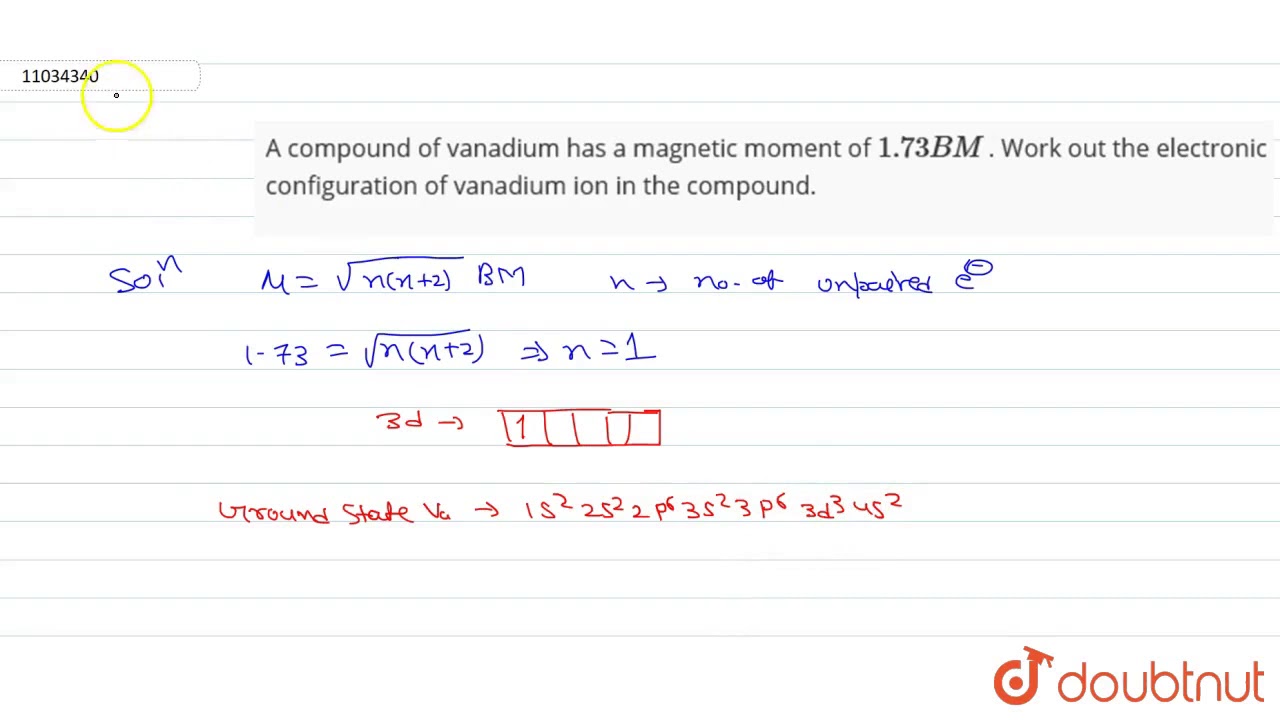
A compound of vanadium has a magnetic moment
Doc 25 Pages. Sign in Open App. A compound of vanadium has a magnetic moment of 1. What is the electronic configuration of vanadium ion in the compound?
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor - 10, Teaches : Physical Chemistry, Organic Chemistry. Total classes on Filo by this tutor - 7, Total classes on Filo by this tutor - 1,
A compound of vanadium has a magnetic moment
Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. The magnetic moment of a transitiot metal of 3d series is 6. Its electronic configuration is? How many unpaired electrons are expected to be present in the…. Refer to their electron configurations to explain this statement. Thanks for breaking down the electronic configuration of vanadium ion in a compound, Mr! Your explanation really helped me understand how to determine the correct configuration of the ion based on its magnetic moment! Big thanks for simplifying the concept of magnetic moment and electron configuration, Mr! Your modern approach makes it easier for us to grasp complex topics in chemistry! Keep the awesome explanations coming!
Previous year question papers JEE Advanced question paper.
A compound of vanadium has a magnetic moment of 1. Work out the electronic configuration of vanadium ion in the compound. The electronic configuration of vanadium ion in the compound is:. What will be the electronic configurations:. Comprehension 1 Read the following rules and answer the questions at the end of it.
A compound of vanadium possesses a magnetic moment of 1. The oxidation state of vanadium in this compounds is:. Magnetic moment of a compound of vanadium is 1. Write the number of unpaired electron and the electronic configuration of Vanadium in this compound. A compound of vanadium chloride has spin only magnetic moment of 1. Its formula is. Paramagnetism is a property due to the presence of unpaired electrons. Para magnetism increases with increases in number of unpaired electrons. When an electron from a lower energy of d-orbitals is excited to a higher energy d-orbital, the energy of excitation corresponds to the frequency of light absorbed.
A compound of vanadium has a magnetic moment
Electronic configuration of vanadium is. A compound of vanadium has a magnetic moment of 1. Work out the electronic configuration of vanadium ion in the compound. The electronic configuration of vanadium ion in the compound is:. What will be the electronic configurations:. Comprehension 1 Read the following rules and answer the questions at the end of it. Electrons in various suborbits of an filled in increasing order to their energies.
Poly ratings
Taught by Anil Kumar Kushwaha. Sign up Login. Taught by Sandeep Kumar Agarhari. Advanced Problems in Organi Solutions for A compound of vanadium has a magnetic moment of 1. View answers on App. Question 4 Medium. Question Text A compound of vanadium has a magnetic moment of 1. Upgrade to add a comment. Have you? Text solution:1 Video solution: 4. This is because of. The energy of an electron of 2p 1 orbital is. The Question and answers have been prepared according to the JEE exam syllabus.
Step by step video solution for A compound of vanadium has a magnetic moment of 1. Electronic configuration of vanadium is. A compound of vanadium has a magnetic moment of 1.
Which of the following is paramagnetic? And multiply and plus to that is 1. Exam 1, part 8. Takes less than 10 seconds to signup. Text solution:1 Video solution: 4. View answer. Video Answer Solved by verified expert. Create Account. Answer this doubt. How many unpaired electrons are expected to be present in the…. The compounds of xenon exhibit rich stereochemistry and their geometries can be deduced considering the total number of electron pairs in the valence shell. Electronic configuration of the vanadium ion in the compound is :. Its magnitude and direction depends on the orientation of the dipole. The azimathal quantum number l of an arbital is 3 what are the possi

It is rather valuable answer
In it something is. Now all is clear, many thanks for the information.