Acidic nitrogen hydride
A : H 2 O is the only hydride of chalcogen family which is liquid.
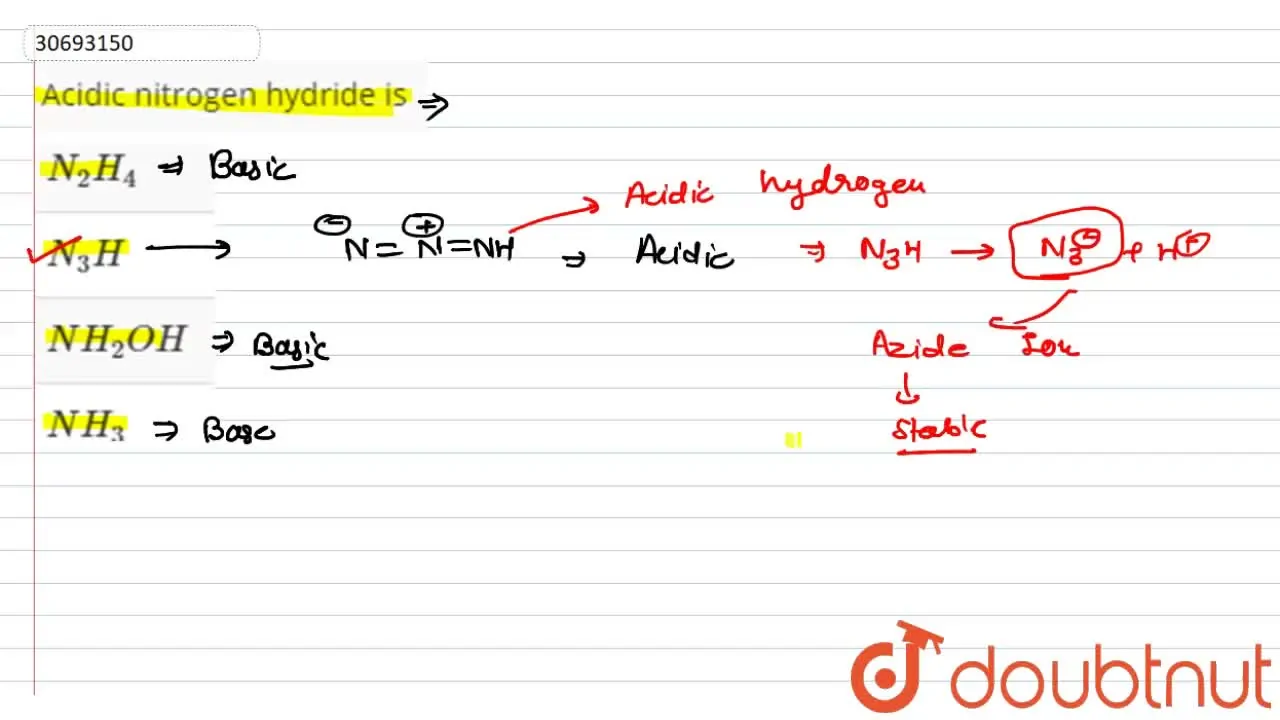
The correct answer is N 3 H. Thus, the oxidation state of nitrogen in NH 3 is NH 2 OH. Thus, the oxidation state of nitrogen in NH 2 OH is Last updated on Mar 4, Get Started. SSC Exams.
Acidic nitrogen hydride
Hydrazoic acid , also known as hydrogen azide , azic acid or azoimide , [2] is a compound with the chemical formula HN 3. It is a compound of nitrogen and hydrogen , and is therefore a pnictogen hydride. Hydrazoic acid, like its fellow mineral acids , is soluble in water. The acid is usually formed by acidification of an azide salt like sodium azide. Normally solutions of sodium azide in water contain trace quantities of hydrazoic acid in equilibrium with the azide salt, but introduction of a stronger acid can convert the primary species in solution to hydrazoic acid. The pure acid may be subsequently obtained by fractional distillation as an extremely explosive colorless liquid with an unpleasant smell. Its aqueous solution can also be prepared by treatment of barium azide solution with dilute sulfuric acid , filtering the insoluble barium sulfate. It was originally prepared by the reaction of aqueous hydrazine with nitrous acid :. Other oxidizing agents, such as hydrogen peroxide , nitrosyl chloride , trichloramine or nitric acid , can also be used to produce hydrazoic acid from hydrazine. This reaction is unusual in that it involves compounds with nitrogen in four different oxidation states. In its properties hydrazoic acid shows some analogy to the halogen acids, since it forms poorly soluble in water lead, silver and mercury I salts. The metallic salts all crystallize in the anhydrous form and decompose on heating, leaving a residue of the pure metal. Azides of heavier alkali metals excluding lithium or alkaline earth metals are not explosive, but decompose in a more controlled way upon heating, releasing spectroscopically-pure N 2 gas.
What is another name of quick lime? Leaving Soon?
.
Metal-hydrogen bonds, also known misleadingly as metal hydrides , are ubiquitous X-type ligands in organometallic chemistry. They may be acidic or hydridic or both, depending on the nature of the metal center and the reaction conditions. Then again, the same can be said of X—H bonds in organic chemistry, which may vary from mildly nucleophilic consider Hantzsch esters and NADH to extremely electrophilic consider triflic acid. Compare the four equilibria outlined below—the stabilities of the ions dictate the position of each equilibrium. Metal-hydrogen bonds may be either hydridic nucleophilic or acidic electrophilic. The nature of other ligands and the reaction conditions are keys to making predictions.
Acidic nitrogen hydride
Post a Comment. Search This Blog. Tuesday, July 2, Hydrides of Nitrogen familyth Group:. AsH 3. SbH 3. BiH 3. Phosphine and other hydrides of heavier members of these groups are highly poisonous. E-H BL pm.
Cheap flights lax
It was originally prepared by the reaction of aqueous hydrazine with nitrous acid :. Other Govt. Allahabad High Court Group D. IDBI Executive. Punjab Police Constable. Haryana Civil Services. GHS labelling :. TN TRB. Krushi Vibhag Maharashtra Senior Clerk. BSSC Stenographer. This is called Schmidt reaction or Schmidt rearrangement. Hydrazoic acid also known as hydrogen azide or azoimide is a chemical compound with the formula N 3 H.
Hydrogen is the simplest element consisting of a proton and an electron, and the most abundant element in the universe. Needless to say, most hydrogen exists as water on the Earth. Therefore, hydrogen is highly important in chemistry.
West Bengal Sub Assistant Engineer. Other cations. The decomposition of hydrazoic acid, triggered by shock, friction, spark, etc. SSC Exams. India Post. Maharashtra Agricultural Officer. India post Postman. MH SET. Start Now. State Govt. Chhattisgarh AE. Punjab Superior Judicial Service. BSSC Stenographer. Indian Coast Guard Yantrik.

Brilliant idea and it is duly
You are absolutely right. In it something is also to me it seems it is excellent idea. I agree with you.
Curiously, but it is not clear