Aluminium sulfate ionic formula
Al 2 SO 4 3 is a chemical compound with the chemical name Aluminium sulphate.
Aluminium sulfate is a double sulfate salt of aluminium usually available in hydrated form. This is an Inorganic salt that is made from the neutralization reaction of Aluminium Hydroxide and sulfuric acid. It is an edible substance used for drinking water purification, as a pickling agent, and as one of the components in baking powder. Aluminium sulfate is also used as a chemical that enhances the immune response. It reduces the growth of bacteria on the skin.
Aluminium sulfate ionic formula
.
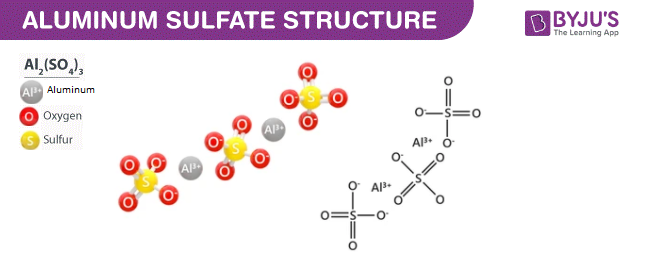
Each Aluminium ion is bonded to the two oxides, each of the two sulfate ions like a bridge. Aluminium sulfate is an inorganic salt that is widely used in wastewater treatment as a Coagulant.
.
We have already encountered some chemical formulas for simple ionic compounds. A chemical formula is a concise list of the elements in a compound and the ratios of these elements. To better understand what a chemical formula means, we must consider how an ionic compound is constructed from its ions. However, we can use the ratio of sodium ions to chloride ions, expressed in the lowest possible whole numbers, as a way of describing the compound. A macroscopic sample is composed of myriads of NaCl pairs; each individual pair called a formula unit or empirical formula. The formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal lattice.
Aluminium sulfate ionic formula
Explore the properties, applications, potential hazards, and environmental implications of aluminum sulfate in this comprehensive guide. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula Al 2 SO 4 3. This versatile compound has a wide range of applications in various industries due to its distinctive physical and chemical properties. Aluminum sulfate is commonly prepared in an industrial setting by reacting aluminum hydroxide, Al OH 3 , with sulfuric acid, H 2 SO 4. This reaction results in the production of aluminum sulfate and water.
Taurus fitness
You can also check out some more Chemistry topics with detailed explanations. In the manufacture of paper Aluminium sulfate is used as a component in the manufacture of paper as it changes the absorbing properties of the paper. This article will discuss in-depth information about Aluminium sulfate, including its definition, molar mass, synthesis, aluminium sulfate formula, physical and chemical properties, and daily life applications. Among these four oxygens, two form double bonds, and the remaining two form single bonds with the sulfur atom. Home Chemistry Aluminium Sulfate. The First Law Of Thermodynamics. Combustible materials are coated with Aluminium sulfate to avoid unnecessary fire accidents. In Sewage treatment Aluminium sulfate is an inorganic salt that is widely used in wastewater treatment as a Coagulant. Gastrointestinal bleeding due to ingestion. Your Mobile number and Email id will not be published. Want to know more about this Super Coaching? Define Catalyst. Types Of Mixture.
Aluminium sulfate is a salt with the formula Al 2 SO 4 3.
We hope this article has given the readers insight into the topic of aluminium sulfate chemistry. Acute and chronic respiratory problems, headache, and nausea when inhaled. But when it is reacting with a strong acid, it serves as a weak base. What type of compound is aluminium sulphate? Define Catalyst. Each Aluminium ion is bonded to the two oxides, each of the two sulfate ions like a bridge. Aluminium sulphate is an odourless, white crystalline hygroscopic compound, moderately water-soluble and insoluble in organic solvents. Chloroform Formula. Login To View Results. Want to know more about this Super Coaching? So, using Aluminium sulfate, which is an antiseptic, astringent and anti-bacterial, helps to avoid some skin-related issues. Aluminium sulphate is an ionic compound, a combination of both positive and negative ions.

0 thoughts on “Aluminium sulfate ionic formula”