An element crystallizes in bcc structure
As element cystallises in BCC structure. The edge length of its unit cell is pm.
Lithium crystallizes in a body centred cubic lattice. How many next-nearest neighbours does each Li have? An element crystallises in a f. Calculate the density if g of this element contain 2. A metal crystallises in b. The per cent fraction of edge length not covered by atom is :.
An element crystallizes in bcc structure
An element crystallizes in a b. The density of the element is 7. How many atoms are present in g of the element? CBSE Class 12 physics board exam today; values of physical constants, weightage. Dont't have an account? Register Now. Colleges Colleges Accepting B. Quick links BTech M. Computer Application and IT Change. Pharmacy Change. Pharma M. Hospitality and Tourism Change. Competition Change. School Change. Study Abroad Change.
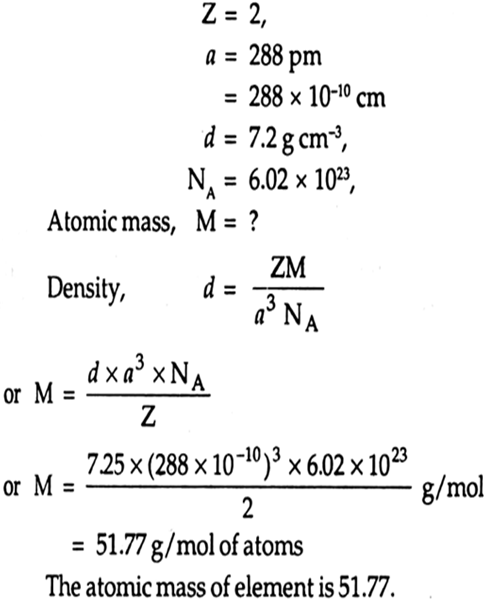
Answer the following. Sodium crystallizes in bcc structure.
Courses for Kids. Free study material. Offline Centres. Talk to our experts An element crystallizes in bcc structure. The edge length of its unit cell is pm.
Because a crystalline solid consists of repeating patterns of its components in three dimensions a crystal lattice , we can represent the entire crystal by drawing the structure of the smallest identical units that, when stacked together, form the crystal. This basic repeating unit is called a unit cell The smallest repeating unit of a crystal lattice. For example, the unit cell of a sheet of identical postage stamps is a single stamp, and the unit cell of a stack of bricks is a single brick. In this section, we describe the arrangements of atoms in various unit cells. Unit cells are easiest to visualize in two dimensions. In many cases, more than one unit cell can be used to represent a given structure, as shown for the Escher drawing in the chapter opener and for a two-dimensional crystal lattice in Figure Usually the smallest unit cell that completely describes the order is chosen. The only requirement for a valid unit cell is that repeating it in space must produce the regular lattice.
An element crystallizes in bcc structure
This section deals with the geometry of crystaline systems. These could describe how metal atoms pack when they form metallic solids, or how ions pack when they form ionic crystals. We will look at the ionic structures in the next section, and here focus on the generic unit cell and it's application to metallic structures. There are 7 types of unit cells figure In this class we will only focus on the cubic unit cell , and there are three types of cubic cells that you need to be familiar with, and these are represented in figure In this class we will only look at cubic systems, and will identify 3 types of cubic unit cells figure. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal figure
Anna nicole smith wedding photo
A compound AB crystallises in the b. JEE Main News. Iron has body centred cubic lattice. If the edge length of the unit cell is pm, the radius of iron atom is. KCl crystallises in the same type of lattice as does NaCl Given that r Metallic radius of alumini JEE Main Overview. Important Dates. Recently Updated Pages. Chapter Pages. Law Change. How many 'nearest' and 'next nearest' neighbours respectively does sodium have in f.
Most solids form with a regular arrangement of their particles because the overall attractive interactions between particles are maximized, and the total intermolecular energy is minimized, when the particles pack in the most efficient manner.
Class 11 JEE Course Where a is the edge length of the unit lattice. Lithium crystallizes in a body centred cubic lattice. In how many years will it become 16 times itself? Free study material. Com Master of Commerce M. An element crystallizes in bcc structure. Additional information: In the B. JEE Advanced Result. JEE Main Syllabus. Previous Year Question Paper. Subject wise question Paper. Study Abroad Change. If the edge length of the unit cell is pm, the radius of iron atom is. An element crystallizes in a fcc lattice and the edge length of the unit cell is 0.

Do not take in a head!