Aqua solution of borax is
Key Points. Additional Information.
Reason R Acetate ion undergoes atomic hydrolysis. An aqueous solution of N a 2 C O 3 is alkaline? Why is an aqueous solution of borax slightly alkaline? Aqueous solution of sodium carbonate is alkaline due to. Aqueous solution of sodium carbona alkaline due to. The aqueous solution of borax turns red litmus to. Explain why an aqueous solution of ammonium chloride is acidic in nature.
Aqua solution of borax is
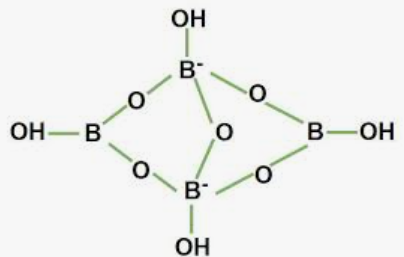
Wiki User. When borax is dissolved in water it ionizes and hydrolysis of borate ion gives OH the hydroxyl ion and boric acid. An aqueous solution is one where water is the solvent. The nature of the solute is not relevant. Yes its aqueous solution is acidic in nature due to hydrolysis of salt. It is a neutral salt but its aqueous solution is acidic in nature. This solution is called water solution or aqueous solution. Yes, this solution NH4OH, ammonium hydroxide is alkaline. NB The word 'aqueous' comes from Latin ; 'aqua' meaning 'water'. Aqueous solutions are solutions in which water is the solvent. Anything that dissolves in water forms an aqueous solution. The term "aqueous" stands for the latin word "aqua" which means water. Tags Borax sodium borate Subjects. Log in. Study now See answer 1.
UP Police Workshop Staff.
When aqueous solution of borax is acidified with hydrochloric acid, a white crystalline solid is formed which is soapy to touch. Is this solid acidic or basic in nature? Why is an aqueous solution of borax slightly alkaline? What is the nature of an aqueous solution of borax? Which of the following compounds is formed by addition of mineral acid to an aqueous solution of borax? State true or false Aqueous solutions of borax acts as a buffer. On the addition of mineral acid to an aqueous solution of borax, the compound formed is:.
Courses for Kids. Free study material. Offline Centres. Talk to our experts Last updated date: 14th Mar Study Material.
Aqua solution of borax is
Borax, also known as sodium borate or sodium tetraborate, is a naturally occurring mineral that has been used for various purposes throughout history. It is composed of boron , sodium, oxygen, and water molecules. Origin and Occurrence : Borax is typically found in dry lake beds, salt flats, and sedimentary rocks. It forms through the evaporation of water in arid regions, leading to the concentration of boron compounds. One of the most famous and abundant sources of borax is the Searles Lake in California, United States. Historical Uses : The history of borax usage dates back thousands of years. Ancient civilizations, such as the Egyptians and Romans, used borax for various purposes, including as a cleaning agent, a flux to assist with soldering metals, and as a preservative for food. It was also used in traditional medicine and in the production of glass and ceramics. Modern Applications : In the modern era, borax continues to have numerous applications across various industries:. Safety Considerations: While borax has a wide range of applications, it is essential to handle it with care.
Pretty little.liars hanna
Maharashtra Nagar Parishad Fire Officer. MP Vyapam Sub Engineer. UP Police Jail Warder. Speed of sound is highest in which medium? CIL MT. Give equation. CWC Junior Superintendent. SBI SO. The cooking gas is mainly a mixture of the following two gases:. Haryana Police. Smoke is a. Engineering Recruitment Exams. An aqueous solution of N a 2 C O 3 is alkaline? Rajasthan High Court District Judge.
Name the two gases from which water is formed.
TN Forest Guard. Nainital Bank Clerk. Bihar Elementary Teacher. Chandigarh Police Constable. Odisha Amin. The term "aqueous" stands for the latin word "aqua" which means water. Rajasthan SET. Punjab Police Constable. Gujarat Metro Maintainer. Why aqueous solution of borax is alkaline? At freezing point of solution.

The happiness to me has changed!