Average atomic mass of sulfur
The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances.
The natural isotopes of sulfur are listed below: 32 S, Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties.
Average atomic mass of sulfur
Submitted by Jill O. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Calculate the atomic mass of sulfur if the four common isotopes of sulfur have masses of Calculate the atomic mass of sulfur symbol S , using data from the table below. Four isotopes of sulfur are sulfur, sulfur, sulfur, and sulfur Abundances for the first three isotopes are What is the average atomic mass of sulfur? Report the answer to five significant figures with units. Calculate the average atomic mass of sulfur if Sulfur has four naturally occurring isotopes, listed in the table below: Determine the number of neutrons for each isotope, and identify which isotope is the most abundant. Isotope Isotope mass u Naturally occurring europium Eu consists of two isotopes with a mass of and
Intro to Hydrocarbons. Group 1A and 2A Reactions. Kinetic Molecular Theory.
Isotope Atomic mass Da Isotopic abundance amount fraction 32 S Isotopes of sulfur are fractionated by various chemical, physical, and biological processes. The major variations in the atomic weight of sulfur on earth are caused by kinetic isotope fractionations accompanying microbial oxidation-reduction reactions such as bacterial reduction of aqueous sulfate, in which the residual unreacted substrate is gradually depleted in the lighter isotopes, which react more rapidly. Over geologic time, processes such as these have resulted in major reservoirs of terrestrial sulfur with different atomic weights: oxidized forms such as marine sulfate commonly being heavy in comparison with the bulk earth and the majority of reduced forms such as organic sulfur and sulfide. The radioactive isotope 35 S is produced by cosmic-ray interactions with 40 Ar in the atmosphere and decays to 35 Cl with a half-life of 87 days. Sulfur was known as brenne stone for "combustible stone" from which brim-stone is derived.
For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26, respectively. These isotopes can be identified as 24 Mg, 25 Mg, and 26 Mg. They differ only because a 24 Mg atom has 12 neutrons in its nucleus, a 25 Mg atom has 13 neutrons, and a 26 Mg has 14 neutrons. Note that in addition to standard names and symbols, the isotopes of hydrogen are often referred to using common names and accompanying symbols. Hydrogen-2, symbolized 2 H, is also called deuterium and sometimes symbolized D. Hydrogen-3, symbolized 3 H, is also called tritium and sometimes symbolized T.
Average atomic mass of sulfur
Sulfur is the 16 th element of the periodic table. These sulfur facts contain chemical and physical data along with general information and history. Sulfur Periodic Table Cell. Atomic Mass: [ If a single value of the atomic mass is needed, use Electron Configuration: [Ne]3s 2 3p 4 shorthand or 1s 2 2s 2 2p 6 3s 2 3p 4 full.
Twins boxscore
Classification of Matter. Periodic Trend: Electron Affinity. Orientations of D Orbitals. Alcohol Reactions: Dehydration Reactions. Natural variations of sulfur isotopic composition. Upgrade to add a comment. Main Group Elements: Periodic Trends. Table of contents. Naming Esters. Naming Carboxylic Acids. Naming Ketones. Please add your first playlist. Main Group Elements: Bonding Types. Molecular Formula.
Last Updated: September 22, Fact Checked. This article was co-authored by Meredith Juncker, PhD. Her studies are focused on proteins and neurodegenerative diseases.
Weak Titrate-Strong Titrant Curves. Standard Reduction Potentials. Periodic Trend: Effective Nuclear Charge. Sign up Login. How do atomic masses reflect isotope abundances? Ksp: Common Ion Effect. Intro to Hydrocarbons. Conversion Factors. You can reuse this answer Creative Commons License. Meija et al. Naming Cyclic Alkanes. Metric Prefixes. Main Group Elements: Bonding Types. Chemistry of the Nonmetals 0.

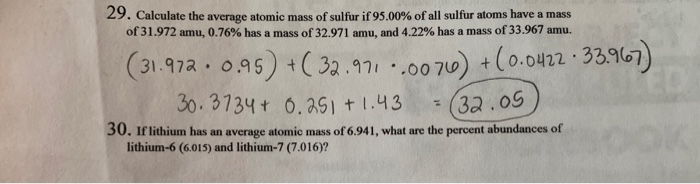
I suggest you to come on a site on which there are many articles on this question.
Thanks for an explanation, the easier, the better �
It is remarkable, this rather valuable message