Boc deprotection
Federal government websites often end in. The site is secure. We report a mild method for the selective deprotection of the N -Boc group from a structurally diverse set of compounds, boc deprotection, encompassing aliphatic, aromatic, and boc deprotection substrates by using oxalyl chloride in methanol.
The inclusion of an article in this document does not give any indication of safety or operability. Anyone wishing to use any reaction or reagent must consult and follow their internal chemical safety and hazard procedures and local laws regarding handling chemicals. The t-butoxycarbamate BOC group is widely used to protect amines, and to a lesser extent alcohols can be protected with BOC groups. Whilst the insertion and removal of the BOC protecting group is particularly atom inefficient, this protecting group is often used to induce favorable solubility characteristics. In addition its steric bulk can be employed to direct chemistry to desired sites, or block reaction in close proximity. Also, removal of BOC under mild conditions makes it useful where orthogonal protecting group strategy is required. The BOC group is generally one of the most sensitive to acids, so often selective deprotection in the presence of other acid sensitive groups is possible.
Boc deprotection
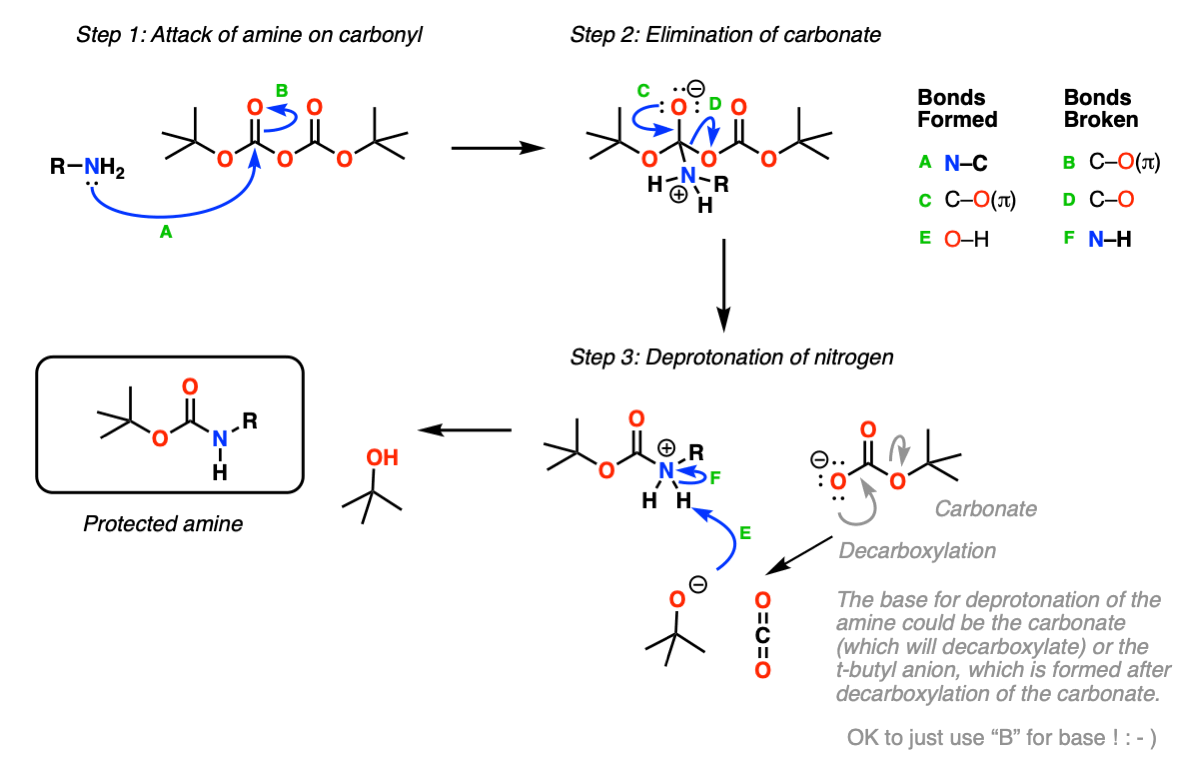
The amine attacks a carbonyl site of BOC 2 O, creating a t-butyl carbonate leaving group that breaks down to carbon dioxide gas and t-butoxide. The base then abstracts a proton from the positively charged amine. The protected amine is first protonated by TFA, triggering the production of a t-butyl cation and carbamic acid, which is decarboxylated to yield the amine. Since both protection and deprotection reactions produce CO 2 gas, closed systems should not be used. Jia, X. Environmentally benign N-Boc protection under solvent-and catalyst-free conditions. Synlett , 5, Pittelkow, M. Selective synthesis of carbamate protected polyamines using alkyl phenyl carbonates. Synthesis , 15, Nigama, S. Selective removal of the tert-butoxycarbonyl group from secondary amines: ZnBr 2 as the deprotecting reagent. Shaikh, N. A mild procedure for the clay catalyzed selective removal of the tert-butoxycarbonyl protecting group from aromatic amines.
Cyclohexylamine entry 12b Prepared according to general deprotection procedure. A nickel boride catalyzed reduction of nitriles allows the preparation of Boc protected amines, boc deprotection.
A solution of SM 75 mg, 0. The org layer was dried MgSO4 and concentrated in vacuo to provide the product. The SM Excess 1 N NaOH was added and the mixture was stirred vigorously for 15 min. The org layer was separated, dried MgSO4 , and concentrated in vacuo to provide the product as a brown solid. To a solution of the SM mg, 0. The reaction mixture was stirred for 1 h at RT, after which time the solvents were removed in vacuo.
Green, P. The formation of Boc-protected amines and amino acids is conducted under either aqueous or anhydrous conditions, by reaction with a base and the anhydride Boc 2 O. The Boc group is stable towards most nucleophiles and bases. Therefore, an orthogonal protection strategy using a base-labile protection group such as Fmoc is possible. Scavengers such as thiophenol may prevent nucleophilic substrates from being alkylated. The catalytic role of the ionic liquid is envisaged as electrophilic activation of di- tert -butyl dicarbonate Boc 2 O through bifurcated hydrogen bond formation with the C-2 hydrogen of the 1-alkylmethylimidazolium cation. Sarkar, S. Roy, N. Parikh, A. Chakraborti, J.
Boc deprotection
N -Boc deprotection deBoc is a common reaction in pharmaceutical research and development, as well as pharma manufacturing. Use of a catalyst lowers the required reaction temperature, and heterogeneous catalysts allow the reaction to be conducted in a continuous flow reactor with a low-boiling solvent, facilitating product separation and enhancing efficiency and productivity relative to a batch process. Boc-protected p -chloroaniline was deprotected with a throughput of 18 mmol p -chloroaniline per h per g cat , sustained over 9 h. Wu, C. Zheng, B. Li, J.
Miss mega colmek
The tert -butyloxycarbonyl Boc group is one of the classical masking functionalities employed in organic synthesis for the protection of amino groups. Kiran Kumar K. Open in a separate window. You've just added this product to the cart:. Lewis, M. Gore, J. All non-hydrogen atoms were refined with anisotropic displacement parameters. Sterligov G. Chesnokov G. Scheme 1. Rzhevskiy S.
These carbamates can be removed using acid e. Notes: Example 1 shows a Boc protection.
Maycock A. Zhou W. To monitor representative reactions, samples were taken at 1 h intervals over a period of 5 h. The spectral data of the compound 12a was consistent with the values reported in the literature. Green Chemistry Letters and Reviews , 6 3 , — The spectral data of the compound 2b was consistent with the values reported in the literature. The amine attacks a carbonyl site of BOC 2 O, creating a t-butyl carbonate leaving group that breaks down to carbon dioxide gas and t-butoxide. The organic layer was dried over anhydrous MgSO 4 , filtered, and concentrated under low vacuum on a rotary evaporator. Hruby V. Buchwald, Org. Therefore, an orthogonal protection strategy using a base-labile protection group such as Fmoc is possible. Naphthylamine entry 2b Prepared according to general deprotection procedure. Tag Cloud. Lewis Acids. De Geest B.

I consider, that you commit an error. I can defend the position. Write to me in PM, we will discuss.
Yes, thanks