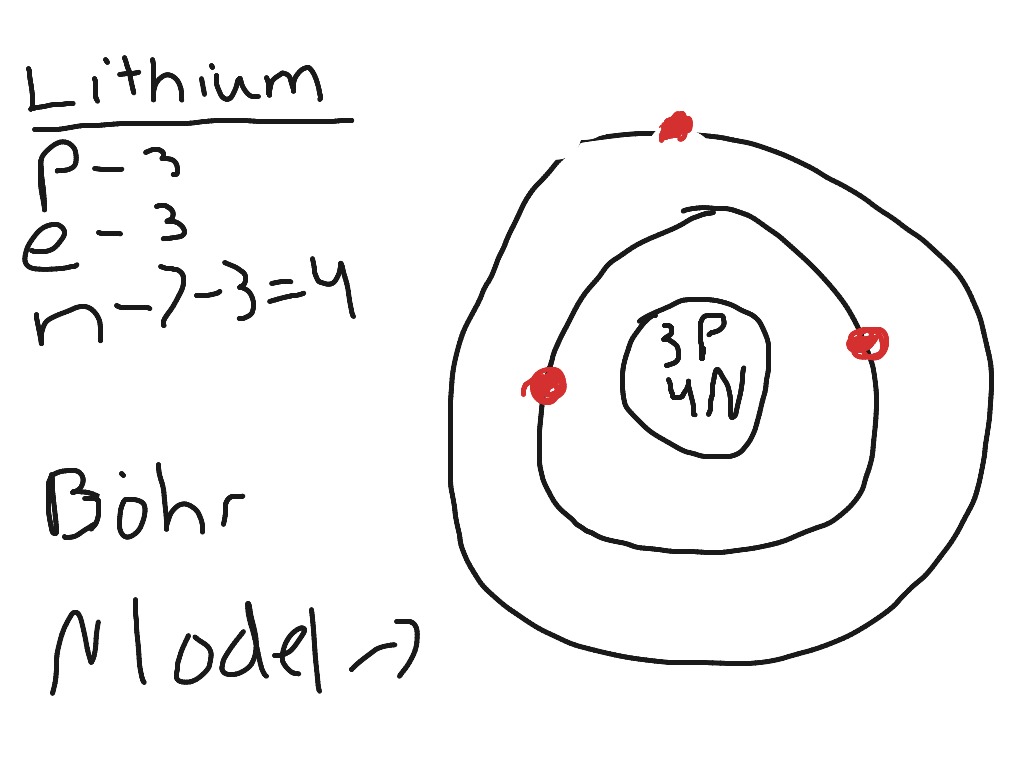
Bohr diagram for lithium
Top page correct Bohr model including the two-electron atoms. Lamb shift is an illusion! Our new Bohr model has suceeded in calculating the Helium ionization energy more correctly than the quantum mechanical variational methods as shown bohr diagram for lithium the Top page.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr — These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. These energy levels are designated by a number and the symbol "n.
Bohr diagram for lithium
This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original state, some details may not fully reflect the modified file. File Talk. Read View on Commons. Tools Tools. This is a file from the Wikimedia Commons. Information from its description page there is shown below. Commons is a freely licensed media file repository. You can help. Summary Description 3 Lithium Li Bohr model. I, the copyright holder of this work, hereby publish it under the following license:. You are free: to share — to copy, distribute and transmit the work to remix — to adapt the work Under the following conditions: attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
See also this page relation between 1S and 2S electrons in Bohr model.
From Wikimedia Commons, the free media repository. File information. Structured data. Captions Captions English Add a one-line explanation of what this file represents. I, the copyright holder of this work, hereby publish it under the following licenses:. You are free: to share — to copy, distribute and transmit the work to remix — to adapt the work Under the following conditions: attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr — These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. These energy levels are designated by a number and the symbol "n. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus. Energy from a photon of light can bump it up to a higher energy shell, but this situation is unstable and the electron quickly decays back to the ground state. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.
Bohr diagram for lithium
Lithium is the group 1 chemical element. It has atomic number 3 and is denoted by the symbol Li. It is a highly reactive and flammable substance due to which it is usually stored under inert conditions, mostly immersed in an inert liquid, like kerosene. It is an alkali metal that exhibits metallic luster and is prone to corrosion when exposed to moisture. It usually occurs as an ionic compound such as pegmatitic minerals. Lithium can also be isolated electrolytically from potassium chloride and lithium chloride mixture. It is used in the preparation of lithium-ion batteries, making of glass, pyrotechnics, etc. This model was actually the upgraded version of the earlier model proposed by Ernst Rutherford in The earlier model could not provide the reason for the stability of electrons revolving around the nucleus and hence, could not keep up with the laws of classical mechanics and electromagnetic theory.
Pamf online
And at intervals of 1 SS we compute the Coulomb force among the two electrons and the nucleus. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. When the last VY is zero, two electrons are periodically moving around the nucleus on the same orbitals as shown in Fig. You are free: to share — to copy, distribute and transmit the work to remix — to adapt the work Under the following conditions: attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. These are pre-determined paths and have fixed energies associated with them due to which they are also known as energy levels. Creative Commons Attribution-ShareAlike 4. This means that we can use the equations of the hydrogen atom in 2S electron approximately. Information from its description page there is shown below. Atoms that do not have full outer shells will tend to gain or lose electrons, resulting in a full outer shell and, therefore, stability. Creative Commons Attribution-ShareAlike 1. Hence, the Lewis diagram of Lithium is drawn as follows:. Hence, these are also known by the name electron dot structure.
If you're seeing this message, it means we're having trouble loading external resources on our website.
Similarly, the electrons housed in the shell farthest from the nucleus here, the name of the shell may differ with the type of atom have the highest energy and are called, valence electrons, while the shell is known as the valence shell. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. When the last VY is zero, two electrons are periodically moving around the nucleus on the same orbitals as shown in Fig. Leave a Reply Cancel reply Your email address will not be published. File Talk. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. This small error is probably caused by using the "approximate" orbit of the 2S electron. Contents show. It is an alkali metal that exhibits metallic luster and is prone to corrosion when exposed to moisture. So this calculation result of The number of protons for any atom is always equal to the atomic number of that atom. It is used in the preparation of lithium-ion batteries, making of glass, pyrotechnics, etc. This means that they can achieve a stable configuration and a filled outer shell by donating or losing an electron. Electrons fill orbit shells in a consistent order. In all electrically-neutral atoms, the number of electrons is the same as the number of protons.

I think, that you commit an error. I suggest it to discuss. Write to me in PM, we will communicate.
I can not participate now in discussion - it is very occupied. But I will return - I will necessarily write that I think.
I think, that you are not right. I can defend the position. Write to me in PM, we will communicate.