Bond order of n2o
The differences here include the number of oxygens attached to nitrogen. Notice the bonding patterns:.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties.
Bond order of n2o
.
Power and Root Functions.
.
N 2 O nitrous oxide has two nitrogen atoms and one oxygen atom. In the N 2 O Lewis structure, there is a triple bond between two nitrogen atoms, and a single bond between nitrogen and oxygen atom. The left nitrogen atom with a triple bond has one lone pair, and the oxygen atom with a single bond has three lone pairs. In the periodic table , nitrogen lies in group 15, and oxygen lies in group Hence, nitrogen has five valence electrons and oxygen has six valence electrons.
Bond order of n2o
This article discusses N2O lewis structure and its hybridization, shape, bond angle, and relevant detailed explanations. N 2 O is covalent molecule. The central N atom is sp hybridized and terminal N and O are sp, and sp 3 hybridized respectively. Being sp hybridization the geometry of Nitrous Oxide is linear. So, the N-N-O bond angle is 0. The central N makes one covalent bond with N and O. The molecule is neutral but in resonance, its show different canonical form, and some of them are charged. The lone pairs reside over N as well as O.
Verdelho vs sauvignon blanc
Multiplication and Division Operations. Naming Alkanes. Chemistry Gas Laws. Radioactive Half-Life. Naming Ethers. Intro to Radioactivity. Truong-Son N. Naming Alcohols. Intro to Henry's Law. Band of Stability: Overview. Mole Fraction. Partial Pressure. Conversion Factors. The Electron Configuration: Quantum Numbers.
But have we ever tried to know more about this gas that can make humans laugh? I guess no! After gaining some knowledge about laughing gas, I decided to share it with you, so that next time we can laugh with knowledge!!
Naming Ionic Hydrates. Standard Reduction Potentials. Millikan Oil Drop Experiment. Chemical Thermodynamics 1h 48m. General Chemistry Strong-Field vs Weak-Field Ligands. The Ideal Gas Law Derivations. Lewis Dot Structures: Ions. Bases Introduction. Alkane Reactions. Titrations: Strong Acid-Strong Base. Reaction Mechanism. Naming Cyclic Alkanes. Introduction to Quantum Mechanics. Bronsted-Lowry Acids and Bases.

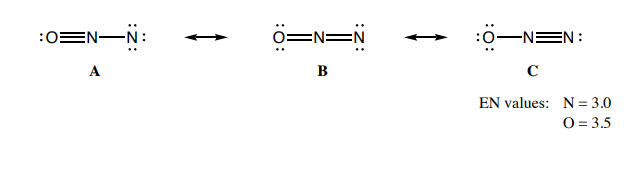
It really surprises.
It is a pity, that now I can not express - I hurry up on job. But I will return - I will necessarily write that I think.