Boron trifluoride shape
The valence bond theory also predicts a planar triangle with hybridisation of one s and two p orbitals used for bonding.
Total: 0. Colourless, heavier-than-air gas with a pungent odour. It forms white fumes in moist air. Boron trifluoride is a colourless , toxic gas with a pungent smell and greater density than air. It forms white smoke during hydrolysis caused by exposure to humid air. It dissolves well in water while forming hydrogen and boric acid. It reacts intensely with metals.
Boron trifluoride shape
The molecular formula of boron trifluoride BF 3 indicates that it has one boron B atom and three fluorine F atoms. Boron is located in Group 13 of the periodic table. It has three valence electrons. Fluorine is located in Group 17 and has seven valence electrons. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest inert gas neighbor, neon. Boron and fluorine will combine to form three B-F single covalent bonds. Boron uses all its three valence electrons to bond with the three fluorine atoms, leaving no lone pairs of electrons. Each fluorine atom will have six lone pairs []. Lewis structure indicates how bonds are formed in BF 3. Boron is the least electronegative of the two atoms. So, it will lie at the center of the molecule. Dash lines represent single covalent bonds. Dots on fluorine represent the lone pairs. According to this theory, the central boron atom has a steric number of 3. Boron has three valence atomic orbitals forming three sp2 hybridized orbitals — one 2s and two 2p orbitals.
Dash lines represent single covalent bonds. You can clearly understand this molecular geometry and nuclear bonding features.
In this article, you will read about BF3 molecular geometry. The inorganic compound is boron trifluoride with formula BF 3. BF 3 is colourless, poisonous gas that has no colour. In damp air, it releases white vapours and is soluble if it is in the form of a colourless liquid i. This plane seems like all peripheral atoms exist in one place. For determining the lewis structure, you need to calculate the total number of valence electrons for the BF 3 molecule.
Boron Trifluoride BF3 is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in different states of matter. In a liquid state, it is a highly soluble substance and in the case of a gaseous state, it is toxic creating white fumes in moist air. Working as a catalyst in the reaction of condensation and esterification, BF3 is used in the production of adhesives and other chemicals and lubricants. It is hazardous to health that damages eyes and skin when comes in contact and very toxic by inhalation. It can also attack plastic and rubber.
Boron trifluoride shape
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U.
Old map of europe 1960
Talk to Our counsellor Give a missed call Trending Topics. Access free live classes and tests on the app. Due to sp 3 hybridization, NF 3 has a pyramidal shape. JEE Application Process. The electron geometry of BF 3 is trigonal planar. If we talk about inconsistency, there is no answer. How Haloform reaction proceed? The geometry of the molecule of BF 3 is known as Trigonal Planar. Frequently Asked Questions. Our website uses cookies to ensure that we give you the best online experience.
The molecular formula of boron trifluoride BF 3 indicates that it has one boron B atom and three fluorine F atoms. Boron is located in Group 13 of the periodic table. It has three valence electrons.
Lewis structure indicates how bonds are formed in BF 3. BF3 Molecular Geometry. Crystalline Polymer. Band Theory. At the BF 3 molecular geometry, there are three B-F bonds. Quantum Numbers. Detailed information. Define back bonding in BF3. Boron is located in Group 13 of the periodic table. Due to this, try adding more than one link to see if the core atom can complete one octet! Learn more. What is the Structure of Borax? References Whatsinsight. Read full. Formation of Complexes.

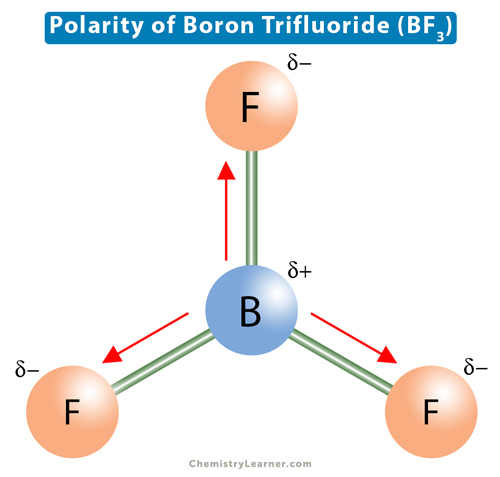
0 thoughts on “Boron trifluoride shape”