C2cl2 lewis structure
Ready to learn how to draw the lewis structure of C2Cl2?
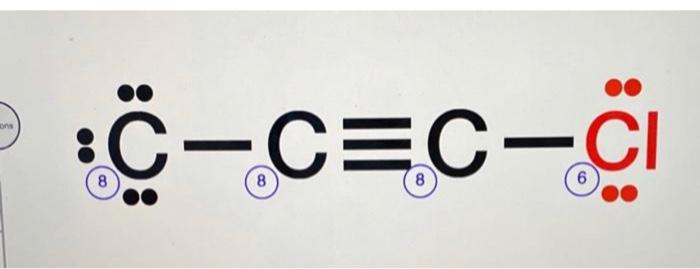
C 2 Cl 2 dichloroacetylene has two carbon atoms and two chlorine atoms. In the C 2 Cl 2 Lewis structure , there is a triple bond between the two carbon atoms, and each carbon is attached with one chlorine atom, and on each chlorine atom, there are three lone pairs. In the periodic table , carbon lies in group 14, and chlorine lies in group Hence, carbon has four valence electrons and chlorine has seven valence electrons. Learn how to find: Carbon valence electrons and Chlorine valence electrons. We have a total of 22 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
C2cl2 lewis structure
C2Cl2 lewis structure has a triple bond between the two Carbon atoms C and a single bond between the Carbon atom C and Chlorine atoms Cl. There are 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in a C2Cl2 molecule , first of all you should know the valence electrons present in carbon atom as well as chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Chlorine is group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. You can see the electronegativity values of carbon atom C and chlorine atom Cl in the above periodic table. If we compare the electronegativity values of carbon C and chlorine Cl then the carbon atom is less electronegative. So here, the carbon atoms C are the center atom and the chlorine atoms Cl are the outside atoms. Now in the C2Cl2 molecule, you have to put the electron pairs between the carbon-carbon atoms and between the carbon-chlorine atoms. This indicates that these atoms are chemically bonded with each other in a C2Cl2 molecule.
Now in the C2Cl2 molecule, you have to put the electron pairs between the carbon-carbon atoms and between the carbon-chlorine atoms.
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The Dichloroethyne molecule contains a total of 3 bond s. There are 3 non-H bond s , 1 multiple bond s , and 1 triple bond s. Images of the chemical structure of Dichloroethyne are given below:. The 2D chemical structure image of Dichloroethyne is also called skeletal formula, which is the standard notation for organic molecules.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons. Nonmetals can form a chemical bond by sharing two electrons. Each atom contributes one electron to the bond. For example, two hydrogen atoms can form a bond, producing a molecule of H 2.
C2cl2 lewis structure
C2Cl2 lewis structure has a triple bond between the two Carbon atoms C and a single bond between the Carbon atom C and Chlorine atoms Cl. There are 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in a C2Cl2 molecule , first of all you should know the valence electrons present in carbon atom as well as chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom.
18624 live running status
Rough sketch of C 2 Cl 2 Lewis structure. Lone pair of left carbon is converted again, and got the stable Lewis structure of C 2 Cl 2. Always start to mark the lone pairs from outside atoms. The C2Cl2 molecule has a total 22 valence electrons and out of these, only 18 valence electrons are used in the above sketch. Please correct it or use other email address. Your email address will not be published. These outer chlorine atoms are forming an octet and hence they are stable. Images of the chemical structure of Dichloroethyne are given below:. Join our subscribers list to get the latest news, updates and special offers delivered directly in your inbox. But after converting one electron pair, one carbon atom is forming an octet but the other carbon atom is still not forming an octet as it has only 6 electrons. In the above structure, you can see that the central atom right carbon forms an octet. Jay Rana.
C 2 Cl 2 dichloroacetylene has two carbon atoms and two chlorine atoms. In the C 2 Cl 2 Lewis structure , there is a triple bond between the two carbon atoms, and each carbon is attached with one chlorine atom, and on each chlorine atom, there are three lone pairs. In the periodic table , carbon lies in group 14, and chlorine lies in group
Hence, the octet rule is satisfied. Both the Chlorine atoms have 3 lone pairs. Below are some application examples that may interest you:. Also, the above structure is more stable than the previous structures. The molecular formula of Dichloroethyne is available in chemical formula page of Dichloroethyne , which identifies each constituent element by its chemical symbol and indicates the proportionate number of atoms of each element. The above structure is not a stable Lewis structure because both carbon atoms have charges. Confirm Valid email address confirmed. By right-clicking the visualization screen, various other options are available including the visualization of van der Waals surface and exporting to an image file. Chlorine is group 17 element on the periodic table. A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together.

You are not right. I am assured. I can defend the position. Write to me in PM, we will communicate.
In my opinion you are not right.