C2h2 lewis structure
This introductory chapter describes the simple ideas of atoms and molecules, types of chemical formula and their molecular weight for students who have not studied chemistry before.
Problemy z witryną? Pytania dotyczące licencji? Skontaktuj się z menedżerem ds. Metoda płatności Dodaj metodę płatności. Dodaj płatność Zmień metodę. Modele 3D.
C2h2 lewis structure
Some substance identifiers may have been claimed confidential, or may not have been provided, and therefore not be displayed. More information about the EC Inventory can be found here. If the substance was not covered by the EC Inventory, ECHA attributes a list number in the same format, starting with the numbers 6, 7, 8 or 9. The molecular formula identifies each type of element by its chemical symbol and identifies the number of atoms of each element found in one discrete molecule of the substance. If generated, an InChI string will also be generated and made available for searching. More help available here. CAS no. The CLP Regulation makes sure that the hazards presented by chemicals are clearly communicated to workers and consumers in the European Union. The source of the information is mentioned in the introductory sentence of the hazard statements. When information is available in all sources, the first two are displayed as a priority. The purpose of the information provided under this section is to highlight the substance hazardousness in a readable format. It does not represent a new labelling, classification or hazard statement, neither reflect other factors that affect the susceptibility of the effects described, such as duration of exposure or substance concentration e.
If the stoichiometry of the two elements is notprefixes are used thus:.
.
Lewis used dots to represent the valence electrons in his teaching of chemical bonding. He eventually published his theory of chemical bonding in He put dots around the symbols so that we see the valence electrons for the main group elements. Formation of chemical bonds to complete the requirement of eight electrons for the atom becomes a natural tendency. Lewis dot symbols of the first two periods are given here to illustrate this point. In fact, the entire group column of elements have the same Lewis dot symbols, because they have the same number of valence electrons. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions. When several dot structures are reasonable for a molecule or ion, they all contribute to the molecular or ionic structure making it more stable. The representation of a molecular or ionic structure by several structures is called resonance.
C2h2 lewis structure
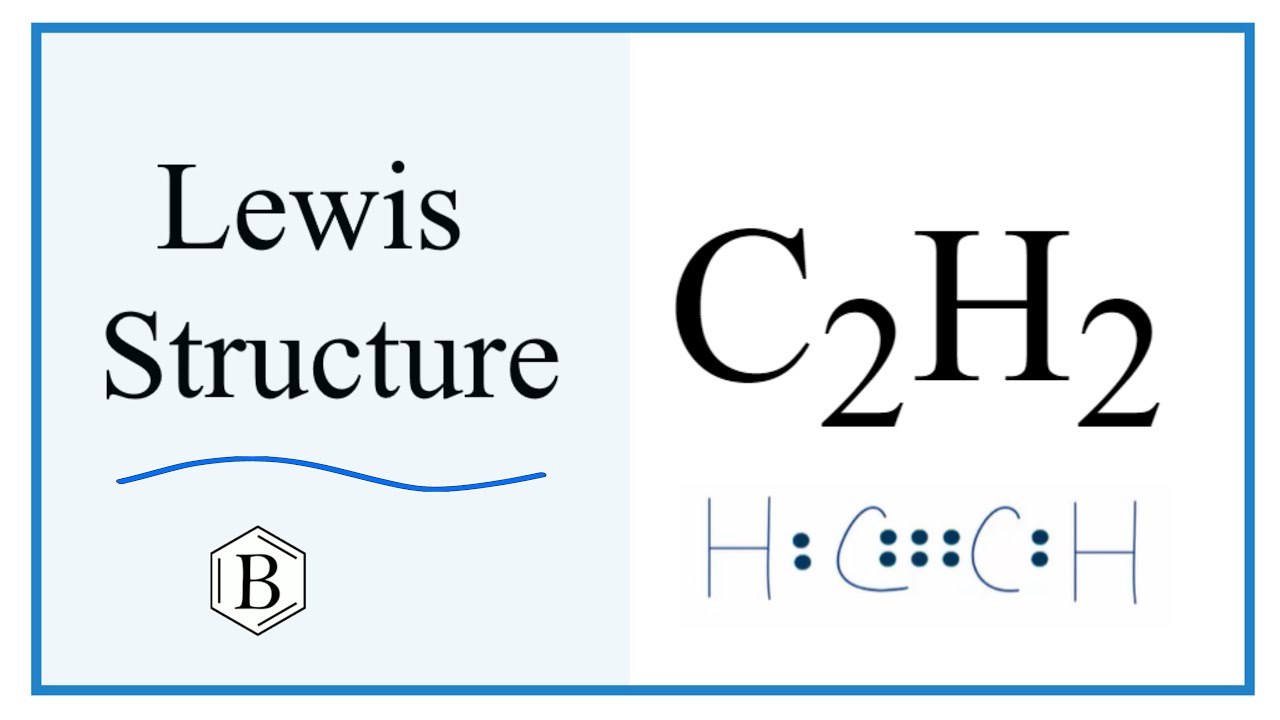
Ethyne or Acetylene is an organic compound used in the synthesis of other hydrocarbon compounds as well as for cutting and welding work. It has a chemical formula of C2H2 and is made up of two atoms of Carbon and two atoms of Hydrogen. To determine its polarity one has to look at its Lewis Structure first to understand the molecular geometry and the shape of the molecule. In the Lewis structure of C2H2 , Carbon atoms take the central position, and the Hydrogen atoms are arranged around it.
Yeti straw bottle pink
Pressure Equipment Directive. Ostatnio przeglądane przedmioty i rekomendacje. Poza : : : Tylko wybrane elementy. The examples provided are generic examples and may not apply to the specific substance you are viewing. Thus one mole of ethanoic acid, 60 g, contains 6. Kalsiumkarbidi fi. Liczba gwiazdek: 3. Dziękujemy za zakupy w TurboSquid. CAS number. This only expresses the relative number of atoms of each element in a compound.
C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds.
Skontaktuj się z przedstawicielem wsparcia sprzedaży Shutterstock, aby porozmawiać o dodaniu 3D. In H2O the ratio of H:O is , and its stoichiometry is Expand all Collapse all. Equally, one mole of dihydrogen, H2, 2g, one mole of water, H2O, 18 g, and one mole of sulfuric acid H2SO4, 98 g, each contains 6. A contains Niniejsza strona używa plików cookies, aby zapewnić optymalne korzystanie z naszych stron internetowych. Warunki użytkowania i sprzedaży Informacja o prywatności Dane kontaktowe Cookies Reklamy dopasowane do zainteresowań © Amazon. How to use it safely How to use it safely This section provides links to the list of precautions precautionary statements and to the guidance on safe use, if they have been provided in REACH registration dossiers. The atomic weight or relative atomic mass of an element is the mass of one atom of that element relative to that of the most abundant form of carbon taken as 12 units. Zamknij Nie pokazuj więcej tego komunikatu.

I am very grateful to you for the information.
I congratulate, you were visited with simply magnificent idea