C2h4 sigma and pi bonds
If you're seeing this message, it means we're having trouble loading external resources on our website.
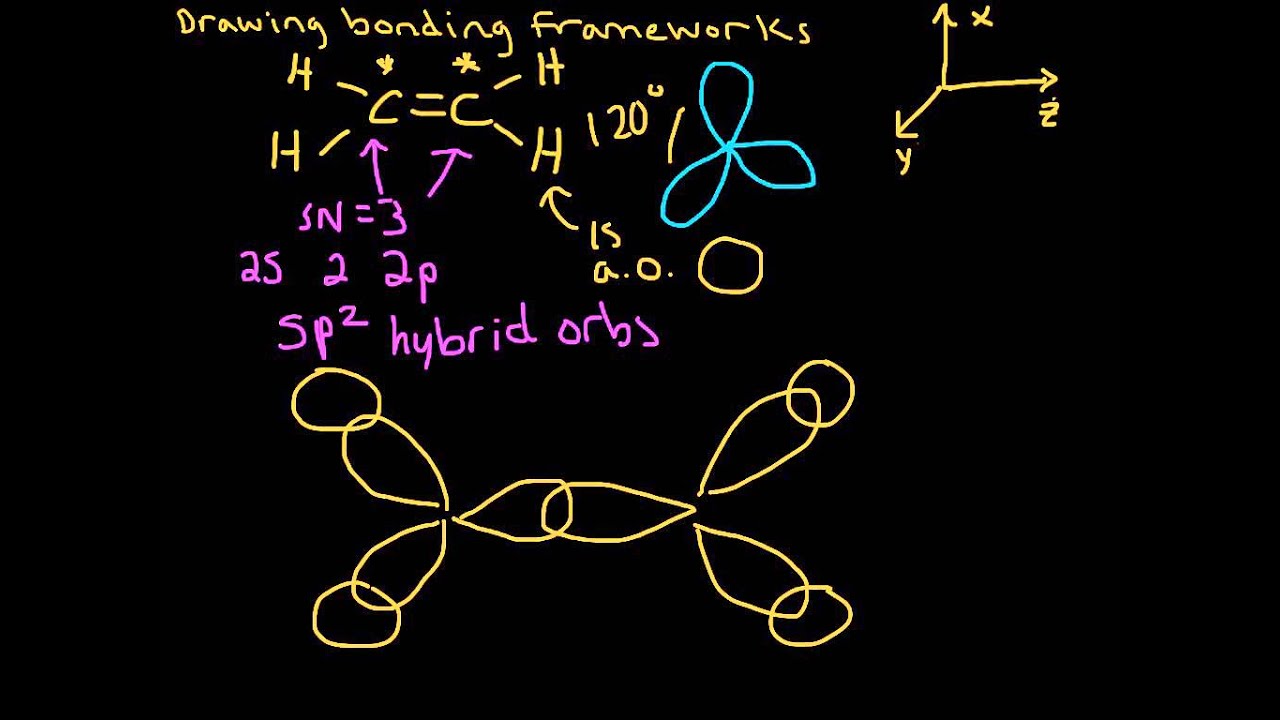
Thus far valence bond theory has been able to describe the bonding in molecules containing only single bonds. However, when molecules contain double or triple bonds the model requires more details. Ethylene commonly knows as ethene , CH 2 CH 2 , is the simplest molecule which contains a carbon carbon double bond. The Lewis structure of ethylene indicates that there are one carbon-carbon double bond and four carbon-hydrogen single bonds. Experimentally, the four carbon-hydrogen bonds in the ethylene molecule have been shown to be identical.
C2h4 sigma and pi bonds
Pages: [ 1 ] Go Down. Topic: Why does C2h4 has pi bonds but C2h6 has sigma only? Read times. I haven't posted an introduction because I was a member here I think the admins just deleted my account because of inactivity, but just for the heck of it I'm a highschooler. PS I clearly understand I should've been giving a better introduction but I literally have 2 months and to cover 2 years worth of studying in it for my GCSE exams im giving accelerated Now coming to the actual question: Here is what I know: S and P orbitals are hybridized to form new orbitals when with c2h4 and c2h6 because carbon only has two unpaired electrons. Why does it form three sp2 orbitals and have one p orbital to make pi bond with another carbon in case of c2h4? Since the number of bonds are the same why not just make four sp3 orbitals as it does in c2h6 and form sigma bonds two with hydrogen and two with carbon? Quote from: vinci on July 31, , AM. Thank you for the explanation. You've given a thorough explanation, it's just my ignorance with the subject that's being a boulder in the way. Hopefully, sooner or later, that will be eliminated. If I try it in the simplest of terms it has got more to do with how ehtylene is formed by the oxidation of ethane.
And now let's draw this carbon. This difference in formation leads to a difference in strength. Now sigma bonds, which are what form when you have a single bond, these are stronger than pi bonds; pi bonds come into play once you start forming double or triple bonds on top of a sigma bond.
When you hear the words sigma and pi bond, you might think of Greek life in college. But actually, sigma and pi bonds are types of covalent bonds. Covalent bonds happen when atoms share electrons. They are found in single, double, and triple bonds. They only exist in double and triple bonds. So, what's the difference between sigma and pi bonds?
We have talked about how covalent bonds are formed through the sharing of a pair of electrons; here we will apply the valence bond theory to explain in more detail how the sharing happens. The valence bond theory describes the covalent bond formed from the overlap of two half-filled atomic orbitals on different atoms. The atomic electron configuration of a hydrogen atom is 1s 1 , meaning that there is one electron which is also the valence electron in the sphere-shaped 1s orbital. When two hydrogen atoms are approaching each other, the two 1s orbitals overlap, allowing the two electrons each H donates 1 electron to pair up for the bonding with the overlapping orbitals. The overall energy changes of the system versus the distance between the two hydrogen nuclei can be summarized in the energy diagram below. When the two atoms are separate, there is no overlap and no interaction. As they are getting closer, orbitals start to overlap, and there is attraction between the nucleus of one atom and the electron of the other atom, so the total energy of the system lowers.
C2h4 sigma and pi bonds
We start with two atomic orbitals: one unhybridized 2p orbital from each carbon. Each contains a single electron. There is increased electron density between the two carbon nuclei in the molecular orbital — it is a bonding interaction. Molecular orbital theory has been very successfully applied to large conjugated systems, especially those containing chains of carbon atoms with alternating single and double bonds. This is, in fact, a more sophisticated version of a free-electron model. This angle suggests that the carbon atoms are sp 2 hybridized, which means that a singly occupied sp 2 orbital on one carbon overlaps with a singly occupied s orbital on each H and a singly occupied sp 2 lobe on the other C.
What tax topic 152 mean
Organic Synthesis. Ozone Depletion. Bonding and Elemental Properties. Paper Chromatography. But what's happening here? It has absolutely nothing to do with any actual heads but instead this difference refers to where the bonding between orbitals actually occurs. Polar and Non-Polar Covalent Bonds. Free Energy. Three atomic orbitals on each carbon — the 2 s , 2 p x and 2 p y — combine to form three sp 2 hybrids, leaving the 2 p z orbital unhybridized. Right, so now you are probably wondering what head-to-head and side-to-side overlap of atomic orbitals even means. Periodic Table.
Forgot password?
This is an s orbital overlapping with an sp2 orbital, but they're kind of overlapping in the direction that they're pointed, or kind of along the direction of each other, of the two atoms. What thought process should I follow to get me from "3 sigma bonds and 1 pi bond" to "2sp2 2sp2 2sp2 2p? Equilibrium Constants. And then you have-- and they have another lobe a little bit on the other side, but I'm not going to draw them. It'll complicate it. Optical Isomerism. Type of Bond Overlapping Atomic Orbitals. Pi bonds tend to be weaker than sigma bonds because the side-by-side overlap the p orbitals give a less effective orbital overlap when compared to the end-to-end orbital overlap of a sigma bond. So you can imagine that this is kind of a Mercedes sign if you drew a circle around it, on its side. Metallic Solids. Rate Equations - The Rate Constant. Topic: Why does C2h4 has pi bonds but C2h6 has sigma only?

0 thoughts on “C2h4 sigma and pi bonds”