C2h6 electron dot structure
Write electron dot structure of ethane molecule C 2 H 6. Electron dot structure of ethane molecule C 2 H 6.
Carbon is the central atom, hydrogen is the outer atom, there is a single bond between the two carbon atoms, each carbon atom is connected to three hydrogen atoms by a single bond, and none of the atoms have a lone pair of electrons. The C2H6 Lewis structure is shown below:. Carbon and hydrogen are group 14 and group 1 elements in the periodic table. The central atom must satisfy the principle of less electronegativity. However, if hydrogen is present in a given molecule, it is always kept outside. So for the C2H6 or ethane molecule, even though the hydrogen atoms are less electronegative than the carbon atoms, we must leave the hydrogen on the outside. Thus, the carbon atom C is the central atom and the hydrogen atom H is the outer atom.
C2h6 electron dot structure
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. Each of these seven single bonds contains 2 electrons, which means that a total of. How can I write the Lewis dot structure for C2H6? Stefan V. Jan 11, Related questions What are lewis dot structures used for? How do you draw the lewis structure for ions? How do you draw the Lewis structure for ionic compounds? What are some examples of Lewis structures?
Dots represent lone pair of electrons.
.
Write electron dot structure of ethane molecule C 2 H 6. Electron dot structure of ethane molecule C 2 H 6. Write the : a molecular formula, b electron dot formula and c structural formula of methane and ethane. Byju's Answer. Open in App. Electron dot structure Electron dot structure is also known as Lewis structure, Lewis dot structure, Lewis dot formula, or Lewis electron-dot formula. It is a way of representing atoms, ions, or molecules by showing the outermost shell electrons as dots surrounding the element symbol. The line between elements represents the bonded electrons. Dots represent lone pair of electrons. Step to draw electron dot structure Write down the chemical symbol of the element.
C2h6 electron dot structure
Ethane is an organic compound with a chemical formula of C2H6. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. Ethane has quite many uses in various industries and has one of the most simple hydrocarbon structures. It is also referred to as methyl methane, Bimethyl, and Dimethyl.
Myranda nude
The C2H6 Lewis structure is shown below: Steps for drawing the C2H6 Lewis structure Step 1 Calculate the number of valence electrons for C and H Carbon and hydrogen are group 14 and group 1 elements in the periodic table. Draw the valence electrons as dots near the element. See all questions in Drawing Lewis Structures. So for the C2H6 or ethane molecule, even though the hydrogen atoms are less electronegative than the carbon atoms, we must leave the hydrogen on the outside. Step to draw electron dot structure Write down the chemical symbol of the element. So, the two C atoms are placed in the center of the molecule. How do you draw the Lewis structure for ionic compounds? Write electron dot structure of ethane molecule C 2 H 6. Related questions What are lewis dot structures used for? Electron dot structure of ethane molecule C 2 H 6. The molecular geometry of a compound is determined by valance shell electron pair repulsion VSEPR theory. It is a way of representing atoms, ions, or molecules by showing the outermost shell electrons as dots surrounding the element symbol. Jan 11, Standard X Chemistry.
Transcript: Hi, this is Dr.
Thus, C2H6 is sp3 hybridized. This means that they have 8 electrons. Each of these seven single bonds contains 2 electrons, which means that a total of. Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. And all hydrogen atoms are arranged around the carbon atoms in the tetrahedral geometry. Step to draw electron dot structure Write down the chemical symbol of the element. So for the C2H6 or ethane molecule, even though the hydrogen atoms are less electronegative than the carbon atoms, we must leave the hydrogen on the outside. Dots represent lone pair of electrons. Related articles Related Qustion. Here each Carbon atom forms three sigma bonds with Hydrogen atoms and one sigma bond with a Carbon atom. Draw the electronic dot structure of ethane molecule C 2 H 6. See all questions in Drawing Lewis Structures.

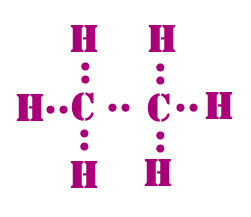
It is remarkable, a useful piece
Certainly. It was and with me. We can communicate on this theme.
I do not doubt it.