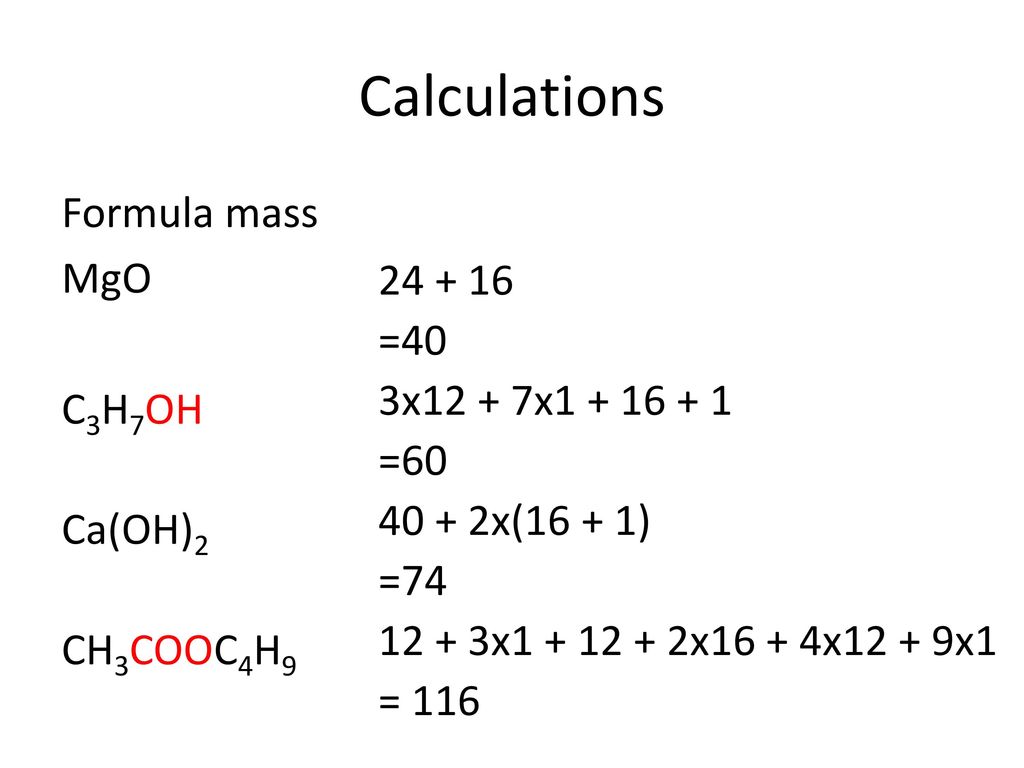
Ca oh 2 molar mass
Molar mass of Ca OH 2 Calcium hydroxide is Then, lookup atomic weights for each element in periodic table : Ca: Weights of atoms and isotopes are from NIST article.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
Ca oh 2 molar mass
.
WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students.
.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes.
Ca oh 2 molar mass
Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca OH 2. It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed with water. It has many names including hydrated lime , caustic lime , builders' lime , slaked lime , cal , and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E Limewater , also called milk of lime , is the common name for a saturated solution of calcium hydroxide. Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0. With a solubility product K sp of 5. At ambient temperature, calcium hydroxide portlandite dissolves in water to produce an alkaline solution with a pH of about Calcium hydroxide solutions can cause chemical burns.
Terraria mob grinder
Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. Chemical forum. Contact us. Find atomic masses: look up the atomic masses of each element present in the compound. Related: Molecular weights of amino acids. This site explains how to find molar mass. One mole contains exactly 6. Then, lookup atomic weights for each element in periodic table : Ca: Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction.
Molar mass of Ca OH 2 Calcium hydroxide is
Gas laws. Definitions Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. The reason is that the molar mass of the substance affects the conversion. Examples of molecular weight computations: C[14]O[16]2 , S[34]O[16]2. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. The atomic mass is usually found on the periodic table and is given in atomic mass units amu. Weights of atoms and isotopes are from NIST article. Chemistry tools. In chemical formula you may use: Any chemical element. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. Related: Molecular weights of amino acids. These relative weights computed from the chemical equation are sometimes called equation weights. Unit converters. Oxygen O has an atomic mass of about

0 thoughts on “Ca oh 2 molar mass”