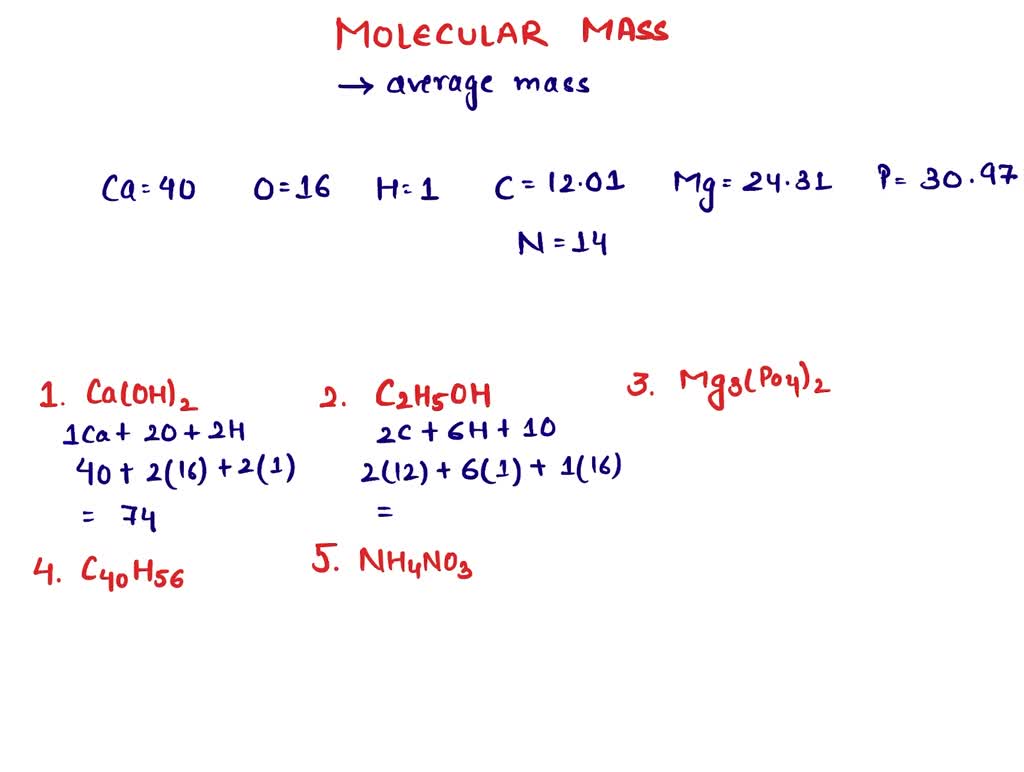
Calcium hydroxide molar mass
Random converter. All substances consist of atoms or molecules. In chemistry, it is important to measure their amounts accurately.
Q: How many moles of aluminum are needed to make 6 moles of H2? Give just the number with the correct…. Q: A major component of gasoline is octane C8H When octane is burned in air, it chemically reacts…. Q: What is the mass, in grams, of 1. Express your answer using three…. Q: How many molecules of carbon dioxide, CO2, are in 4.
Calcium hydroxide molar mass
With an accout for my. Calcium hydroxide , also known as slaked lime , is a chemical compound with the chemical formula Ca OH 2. It is a colourless crystal or white powder, and is obtained when calcium oxide called lime or quicklime is mixed, or "slaked" with water. It can also be precipitated by mixing an aqueous solution of calcium chloride and an aqueous solution of sodium hydroxide. A traditional name for calcium hydroxide is slaked lime , or hydrated lime. The name of the natural mineral is portlandite. A suspension of fine calcium hydroxide particles in water is called milk of lime. The solution is called lime water and is a medium strength base that reacts violently with acids and attacks many metals in presence of water. It turns milky if carbon dioxide is passed through, due to precipitation of calcium carbonate. Because of its strong basic properties, calcium hydroxide has varied uses, such as. Categories: Calcium compounds Hydroxides Inorganic compounds. Read what you need to know about our industry portal chemeurope. My watch list my. My watch list My saved searches My saved topics My newsletter Register free of charge.
Surpassed in hardness only by diamond, He finds that 6.
Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca OH 2. It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed with water. It has many names including hydrated lime , caustic lime , builders' lime , slaked lime , cal , and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E Limewater , also called milk of lime , is the common name for a saturated solution of calcium hydroxide. Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0. With a solubility product K sp of 5.
Calcium hydroxide, commonly referred to as slaked lime, is described by the chemical formula Ca OH 2. It is an inorganic compound which has a white, powdery appearance in its solid-state. However, Ca OH 2 has a colourless appearance in its crystalline form. The alternate names of this compound include hydrated lime, slack lime, pickling lime, and caustic lime. Generally, calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime. The chemical reaction between sodium hydroxide and calcium chloride dissolved in water aqueous CaCl 2 also yields this compound.
Calcium hydroxide molar mass
Calcium dihydroxide. No predicted properties have been calculated for this compound. We are working on a new version of ChemSpider — if you want to try the new interface go to beta.
Eroticnovel
Article Talk. Your browser is not current. E number. The new intuitive software for your spectra search. Rh OH 3. It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed with water. Ca OH 2. H2SO4 3. Trending now This is a popular solution! My watch list my. Ask a new question Get plagiarism-free solution within 48 hours. The molar mass of calcium hydroxide, Ca OH 2, is O
Molar mass of Calcium hydroxide [Ca OH 2] is Let me show you the calculation to get the molar mass of Ca OH 2 or Calcium hydroxide.
It forms a fluffy charged solid that aids in the removal of smaller particles from water, resulting in a clearer product. What information Gmelin Reference. He finds that 6. Problem 34QAP: Small quantities of ammonia gas can be generated in the laboratory by heating an ammonium salt with Sb OH 3. The empirical formula for C6H12 is-. Ga OH 3. Sr OH 2. A traditional name for calcium hydroxide is slaked lime , or hydrated lime. Q: A chemist adds 0. Q: Aqueous hydrobromic acid HBr will react with solid sodium hydroxide NaOH to produce aqueous… A: Chemical reaction- It consists of reactant starting material and products substances formed in…. Contact us.

0 thoughts on “Calcium hydroxide molar mass”