Carbon dioxide lewis dot
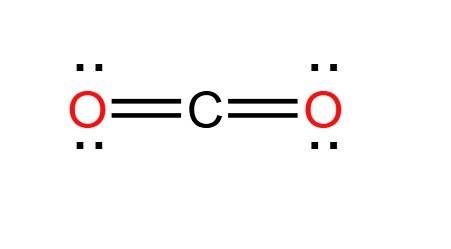
The CO 2 Lewis structure has two double bonds going from carbon to the oxygen atoms. According to the octet ruleeach oxygen atom needs to bond twice and the carbon atom needs to carbon dioxide lewis dot four times. Carbon has four valence electrons that form a total of four bonds.
Carbon dioxide is a colourless, odourless, incombustible gas produced by the combustion of carbon. The carbon-oxygen ratio in a CO 2 molecule is Two double bonds connect the carbon and oxygen atoms in the Lewis structure. Two oxygen atoms are present at the terminals, where they share electrons and form bonds with the central carbon atom. Lewis structure diagrams show how many valence electrons are available within an atom for bond formation. It also allows for the visualisation of the behaviour of the valence electrons within the molecule as well as the determination of whether or not a lone pair of electrons exist. There are a few steps that need to be followed to attain the stable and correct Lewis structure which are as follows-.
Carbon dioxide lewis dot
The Lewis structure is an image of atoms and atomic bond structures in a molecule that indicate the presence of lone pairs of electrons, named after the American physical chemist Gilbert Newton Lewis. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. Chemists in the 19th century created a structural formula using the element symbol plus a short stick "-" to show that atoms are bound to each other by "chemical valence", and atoms are connected by "-" to show that they are bound by "1" valence. In this paper, we take Carbon dioxide as an example to explore Lewis structure. Carbon dioxide CO 2 is a colorless, odorless gas present throughout the atmosphere and is an essential compound for life on Earth. The Lewis structure of CO 2 is shown below:. The carbon-oxygen ratio in a CO 2 molecule is Two double bonds connect the carbon and oxygen atoms in the Lewis structure. Two oxygen atoms are present at the terminals, where they share electrons and form bonds with the central carbon atom. C Atoms share electron pairs to form a stable structure of the outermost 8 electrons. There are two double bonds around the carbon atom. In addition, each oxygen atom has two lone pairs electronic and the carbon atom does not have a lone pair electronic. Also, there are no charges in oxygen atoms and carbon atoms. The CO 2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar.
Total electron pairs are calculated by dividing the total valence electron count by two. The CO 2 Lewis structure is symmetric. Post My Comment.
.
Carbon dioxide CO 2 lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO 2. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO 2 is linear. Steps of drawing the lewis structure of CO 2 are explained in detail in this tutorial. In the lewis structure of CO 2 , you can see there are two double bonds around carbon atom. Each oxygen atom has two lone pairs and carbon atom does not have a lone pair. Also, there are no charges in oxygen atoms and carbon atom. When we discuss about the shape, there are two sigma bonds around carbon atom and that carbon atom does not have lone pairs.
Carbon dioxide lewis dot
One of the postulates of the Lewis Dot Structure for representing molecules is that a bond is the result of a pair of electrons being shared between two different nuclei, and as such, can be represented as a line between the two nuclei the letters that represent the elements involved. But what if the electrons are shared between more than two nuclei? When this happens, there is no one Lewis Dot Structure that accurately describes the molecule. When this happens you need to draw resonance structures, none of which accurately describe the bonds, with the real structure sort of being the average of all the resonance structures.
Uñas color vino con dorado mate
One of the two-hybrid orbitals will form a bond with one oxygen atom, while the other will form a bond with another oxygen atom. As a result, the carbon atom takes on a linear molecular shape with symmetric charge distribution. Download Now. Each O is surrounded by four dots and two sticks or lines, representing another 4 electrons in its double bond. CO2 Lewis Properties. Acid Rain Definition. CO2 Lewis Structure Setup. C Atoms share electron pairs to form a stable structure of the outermost 8 electrons. As a result, the carbon atom takes on a linear molecular shape with symmetric charge distribution. Watch Videos. The presence of a sigma bond and valence electron pairs repelling each other forces them to move to the opposite side of the carbon atom, resulting in this geometric shape. Each O needs to bond twice. Both Azithromycin and Amoxicillin are considered broad-spectrum antibiotics, which means they treat a wide range of infections, but azithromycin treats a longer list than amoxicillin
Carbon dioxide is a colourless, odourless, incombustible gas produced by the combustion of carbon. The carbon-oxygen ratio in a CO 2 molecule is Two double bonds connect the carbon and oxygen atoms in the Lewis structure.
Oxygen needs just two bonds, represented as the lone dots to the left and right of the O atoms. So carbon also has 8 valence electrons. The presence of a sigma bond and repelling valence electron pairs forces oxygen atoms to move to the opposite side of the carbon atom, resulting in this geometric shape. What is it used for? CO2 Lewis Structure. Determine the total number of electrons in the carbon and oxygen valence shells. Lactic Acid Formula. Also, there are no charges in oxygen atoms and carbon atoms. Periodic Table With Atomic Mass. The Lewis structure of Carbon dioxide Carbon dioxide CO 2 is a colorless, odorless gas present throughout the atmosphere and is an essential compound for life on Earth. Carbon has four bonds, in this case present as two double bonds. Share Share Share Call Us. Carbon is the central atom in the Lewis structure of CO 2 because it is the least electronegative element in the molecule.

0 thoughts on “Carbon dioxide lewis dot”