Ch2o lewis structure
If you're seeing this message, it means we're having trouble loading external resources on our website.
Formaldehyde, symbolized as CH 2 O, is a simple and widespread organic compound. This colourless gas consists of two hydrogen atoms, one carbon atom, and one oxygen atom. Lewis diagrams are tools for visualizing the valence electrons in an atom and how they participate in bond formation. They also allow us to see if there are any lone pairs of electrons present. These diagrams are formed by drawing electrons as dots, typically in pairs, around the symbol of the atom.
Ch2o lewis structure
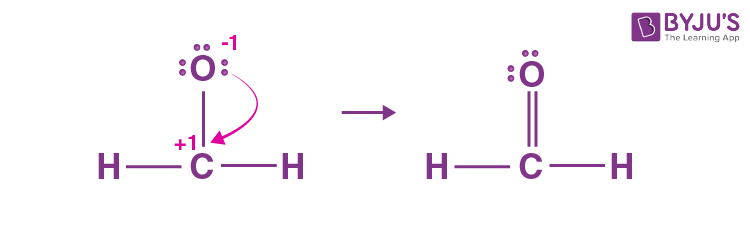
In order to find the total valence electrons in CH2O molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now, you can see the electronegativity values of carbon atom C and oxygen atom O in the above periodic table. If we compare the electronegativity values of carbon C and oxygen O then the carbon atom is less electronegative. This indicates that these atoms are chemically bonded with each other in a CH2O molecule. Here in the sketch of CH2O molecule, you can see that the outer atoms are hydrogen atoms and oxygen atom. These hydrogen atoms and oxygen atom are forming a duplet and octet respectively and hence they are stable.
Unfortunately, the carbon atom is not forming an octet here. The only time we can consider hydrogen as a central atom in some capacity is for small ch2o lewis structure molecules like hydrogen gas, H2, or hydrochloric acid, HCl.
Formaldehyde, symbolized as CH2O, is a simple and widespread organic compound. This colorless gas consists of two hydrogen atoms, one carbon atom, and one oxygen atom. Due to its preservative and disinfectant properties, Formaldehyde is often applied in the industrial production of different products, such as textiles, insulation materials, or cosmetics. However, Formaldehyde is classified by the International Agency for Research on Cancer as carcinogenic, and there are numerous studies about the pernicious health effects that frequent exposure to Formaldehyde can pose to human health. Understanding the structure of Formaldehyde is crucial in comprehending its chemical properties and reactions[1]. Step 1: Determine the total number of valence electrons in the Formaldehyde. Hydrogen, a Group IA element, has one electron in its outer shell.
CH 2 O formaldehyde has one carbon atom, two hydrogen atoms, and one oxygen atom. In the CH 2 O Lewis structure, there are two single bonds around the carbon atom, with two hydrogen atoms attached to it. And the oxygen atom with two lone pairs on it, makes a double bond with the carbon atom. In the periodic table , carbon lies in group 14, hydrogen lies in group 1, and oxygen lies in group Hence, carbon has four valence electrons, hydrogen has one valence electron, and oxygen has six valence electrons. Learn how to find: Carbon valence electrons , Hydrogen valence electrons , and Oxygen valence electrons.
Ch2o lewis structure
This sharing of electrons allowing atoms to "stick" together is the basis of covalent bonding. There is some intermediate distant, generally a bit longer than 0. It is this behavior that Lewis captured in his octet rule. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl 2 , they can each complete their valence shell:. Each chlorine atom now has an octet. The electron pair being shared by the atoms is called a bonding pair ; the other three pairs of electrons on each chlorine atom are called lone pairs.
Supernes
There are 6 steps to draw the Lewis structure of Formaldehyde. Now, you can see the electronegativity values of carbon atom C and oxygen atom O in the above periodic table. Oxygen is a Group VIA element with six electrons in its last shell. Hydrogen, a Group IA element, has one electron in its outer shell. Electrons are more stable existing in pairs as opposed to being unpaired. Why couldn't you take the pair at the top of O and turn that into a bond? CH 2 O is a polar molecule because the difference in electronegativity values between carbon and oxygen leads to charge imbalance and generates a dipole moment in the molecule. For what exactly do you need formaldehyde? The Lewis structure CH2O contains two lone pairs and four bonded pairs two single bonds and one double bond. The shape of the molecule, and the bond angles, are determined by VSEPR theory, not electronegativity. And so, it is drawn this way on a 2D surface to represent these angles. In the valence shells of the HCHO molecule, there are 6 pairs of electrons. So you have seen the above image by now, right? Hydrogen only forms one single bond in these sort of problems.
The Oxygen atom has 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of CH2O.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Based on their placement in the periodic table: Hydrogen, a Group IA element, has one electron in its outer shell. Sorry if this is a silly question but I cannot buy where I live. And then, oxygen, it also is in the second period, and in its second shell it has one, two, three, four, five, six valence electrons. Post My Comment. Read more about our Editorial process. Show preview Show formatting options Post answer. These diagrams are formed by drawing electrons as dots, typically in pairs, around the symbol of the atom. There are charges on the carbon and oxygen atoms in the center. Q: How can you be sure of the amount of covalent electrons, and what if there is 1 extra or 1 less electron in the molecule structure? Well I've just used up the remaining six valence electrons. Minimize charges on atoms by converting lone pairs to bonds to achieve the best Lewis structure.

Clearly, I thank for the information.
Absolutely with you it agree. In it something is also I think, what is it good idea.
It � is impossible.