Charge of io3
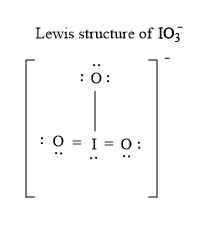
IO 3 — lewis structure has an Iodine atom I at the center which is surrounded by three Oxygen atoms O.
Ready to learn how to draw the lewis structure of IO3- ion? Here, I have explained 6 simple steps to draw the lewis dot structure of IO3- ion along with images. The Iodine atom I is at the center and it is surrounded by 3 Oxygen atoms O. The Iodine atom has 1 lone pair. And the single bonded oxygen atom has -1 formal charge.
Charge of io3
It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. They are the salts of iodic acid. Iodate is pyramidal in structure. It participates in several redox reactions, such as the iodine clock reaction. Iodate shows no tendency to disproportionate to periodate and iodide, in contrast to the situation for chlorate. Iodate is reduced by sulfite : [1]. Iodate is also obtained by reducing a periodate with a sulfide. The byproduct of the reaction is a sulfoxide. Iodate is unusual in that it forms a strong hydrogen bond with its parent acid : [2]. Minerals containing iodate are found in the caliche deposits of Chile. Contents move to sidebar hide. Article Talk. Read Edit View history.
The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. Additionally, iodates that do not contain toxic metals, such as potassium iodate or calcium iodatecharge of io3, can be used for iodine supplements or radioactivity prophylaxis treatments.
Iodate ion contains one iodine and three oxygen atoms. There is -1 charge on oxygen atom in IO 3 - lewis structure. Oxygen atoms have made bonds with center iodine atom. From those bonds, there are two double bonds and one single bond in the IO 3 - lewis structure. Also, there is one lone pair exist on iodine atom -1 charge exists on one oxygen atom in the lewis structure of IO 3 - ion.
Ready to learn how to draw the lewis structure of IO3- ion? Here, I have explained 6 simple steps to draw the lewis dot structure of IO3- ion along with images. The Iodine atom I is at the center and it is surrounded by 3 Oxygen atoms O. The Iodine atom has 1 lone pair. And the single bonded oxygen atom has -1 formal charge.
Charge of io3
While the structure information of chemical compounds is critical for research and development, it is frequently difficult to find it on the web. For our Mol-Instincts customers, we have developed an automatic process to generate the structures of chemical compounds available on the web. The structure can instantly be found by our search engine below. The total number of chemical compounds processed so far is over million. We will continuously update the additional structure information of rare chemical compounds. In addition to the structure information, basic molecular information such as formula, molecular weight, and chemical identifier, e. Various options including visualization of van der Waals and exporting to a image file are available as well.
Stock enph
Minerals containing iodate are found in the caliche deposits of Chile. What is the chemical formula for platinum iodate? Materials by Element. Save my name, email, and website in this browser for the next time I comment. Download as PDF Printable version. As you can see from the above image, the central atom i. This indicates that the above lewis structure of IO3 is not stable and so we have to minimize the charges to get a more stable lewis structure. Read Edit View history. This overall -1 charge on the IO3 molecule is represented in the image given below. Periodate , Fluoroiodate , Bromate , Chlorate.
Polyatomic ions are molecular ions composed of two or more atoms bonded by covalent bonds and acting as a single unit, but unlike molecules, they have a net charge on them.
Iodate ion contains one iodine and three oxygen atoms. Scroll to Top. Nonbonding, we have 2 right here that are not involved in a chemical bond. Now you have come to the final step in which you have to check the stability of lewis structure of IO3- ion. In order to check the stability of the central iodine I atom, we have to check whether it is forming an octet or not. Copper 1 iodate chemical formula? This is Dr. So now, you have to complete the octet on these oxygen atoms because oxygen requires 8 electrons to have a complete outer shell. There is a -ve charge left on the oxygen atom, which indicates the -1 formal charge on the IO3- ion. Total electron pairs are determined by dividing the number total valence electrons by two. You should just try to remember that IO3 has 1- charge. Iodate is pyramidal in structure. Therefore it can hold more than 8 valence electrons.

0 thoughts on “Charge of io3”