Chemosensors
How to publish in this journal.
Open access peer-reviewed chapter. Chemosensors for anions and cations detections have been extensively used in several disciplines, including pharmacology, environmental science, biology, and chemistry. This field which is a division of supramolecular chemistry has been known for more than years. It deals with chemosensors that recognize and detect anions and cations via optical or electrochemical signals. Today, a sustainable variety of chemosensors are established to detect both anions and cations.
Chemosensors
A molecular sensor or chemosensor is a molecular structure organic or inorganic complexes that is used for sensing of an analyte to produce a detectable change or a signal. The application of chemosensors is referred to as chemosensing, which is a form of molecular recognition. All chemosensors are designed to contain a signalling moiety and a recognition moiety , that is connected either directly to each other or through a some kind of connector or a spacer. Chemosensors may also be electrochemically based. Small molecule sensors are related to chemosensors. These are traditionally, however, considered as being structurally simple molecules and reflect the need to form chelating molecules for complexing ions in analytical chemistry. Chemosensors are synthetic analogues of biosensors , the difference being that biosensors incorporate biological receptors such as antibodies, aptamers or large biopolymers. Chemosensors describes molecule of synthetic origin that signal the presence of matter or energy. A chemosensor can be considered as type of an analytical device. Chemosensors are used in everyday life and have been applied to various areas such as in chemistry, biochemistry, immunology, physiology, etc. The signalling moiety acts as a signal transducer , converting the information recognition event between the chemosensor and the analyte into an optical response in a clear and reproducible manner. Most commonly, the change the signal is observed by measuring the various physical properties of the chemosensor, such as the photo-physical properties seen in the absorption or emission , where different wavelengths of the electromagnetic spectrum are used. Colorimetric chemosensors give rise to changes in their absorption properties recorded using ultraviolet—visible spectroscopy , such as in absorption intensity and wavelength or in chirality using circularly polarized light , and CD spectroscopy. In contrast, then in the case of luminescent chemosensors, the detection of an analyte, using fluorescence spectroscopy , gives rise to spectral changes in the fluorescence excitation or in the emission spectra, which are recorded using a fluorimeter.
Chemosensors Yahya thesis of Reference No: Trends in Biotechnology.
.
Open access peer-reviewed chapter. Chemosensors for anions and cations detections have been extensively used in several disciplines, including pharmacology, environmental science, biology, and chemistry. This field which is a division of supramolecular chemistry has been known for more than years. It deals with chemosensors that recognize and detect anions and cations via optical or electrochemical signals. Today, a sustainable variety of chemosensors are established to detect both anions and cations.
Chemosensors
A chemoreceptor , also known as chemosensor , is a specialized sensory receptor which transduces a chemical substance endogenous or induced to generate a biological signal. In bacteria , chemoreceptors are essential in the mediation of chemotaxis. Bacteria utilize complex long helical proteins as chemoreceptors, permitting signals to travel long distances across the cell's membrane. Chemoreceptors allow bacteria to react to chemical stimuli in their environment and regulate their movement accordingly. This is an indicator that chemoreceptors play a heightened role in the sensing of cytosolic signals in archaea. Primary cilia , present in many types of mammalian cells , serve as cellular antennae. Plants have various mechanisms to perceive danger in their environment.
Uber eats promo code adelaide
One of the most valuable response methods for optical readout is fluorescence. The toxicity of certain anions and cations for humans as well as animals has motivated researchers to design chromophores that are selective to a specific anion or cation [ 6 — 8 ]. Quenching by electron transfer between uncharged species leads to the formation of a radical ion pair. The design of the fluorescent chemosensors is given in Figure 2. The Journal of Biological Chemistry. The communication pathway is through electron transfer or energy transfer for such fluorescent chemosensors. Tetrahedron Letters. Read Edit View history. Year SJR 0. In such systems, the sensing event is normally described as being due to changes in the photophysical properties of the chemosensor systems due to chelation induced enhanced fluorescence CHEF , [26] [27] [28] and photoinduced electron transfer PET , [29] [30] [31] mechanisms. In principle the two mechanisms are based on the same idea; the communication pathway is in the form of a through-space electron transfer from the electron rich receptors to the electron deficient fluorophores through space. Journal of the Chemical Society, Chemical Communications 23 : — While the signalling moiety acts as a signal transducer, converting the recognition event into an optical response.
Fluorescent chemosensors for ions and neutral analytes have been widely applied in many diverse fields such as biology, physiology, pharmacology, and environmental sciences. The field of fluorescent chemosensors has been in existence for about years.
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. The essential parts of the fluorescent chemosensors are the recognition and signaling moieties, as given in Figure 1. Hence, a correlation can be made between luminescence intensity, quantum yield and in some cases lifetime and the analyte concentration. Journal of the American Chemical Society. A mini review on organic chemosensors for cation recognition Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research research articles, conference papers and reviews in three year windows vs. Such an effect has been demonstrated for the sensing of anions such as carboxylates and fluorides. An experimental sensor for this compound is again based on PET. Fluorescent chemosensing [ edit ] All chemosensors are designed to contain a signalling moiety and a recognition moiety. ESIPT process is based on a proton transfer from a proton donor hydroxyl or amino unit to an acceptor unit carbonyl oxygen or imine nitrogen atom in the excited state of a fluorophore which is facilitated by an intramolecular hydrogen bond [ 20 ]. Fluorescent chemosensors for copper II ion: Structure, mechanism and application. While converting information into a detectable signal is due to the signaling moiety.

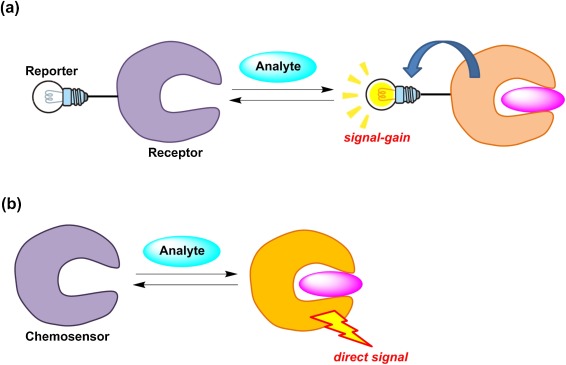
All can be