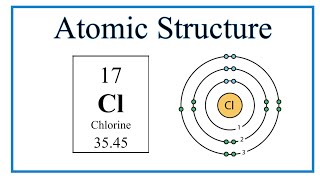
Chlorine bohr model
If you're seeing this message, it means we're having trouble loading external resources on our website.
The two electrons in the first shell should be together not single. This is a Bohr model of a chlorine atom. Some Bohr models pair six of the seven electrons in the third valence shell. This helps to see that one valence electron is available for bonding. From my research, Bohr models of Cl either have the electrons in the second and third shells paired, or they don't have any paired electrons in the first, second, and third shells.
Chlorine bohr model
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr — These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. These energy levels are designated by a number and the symbol "n. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus. Energy from a photon of light can bump it up to a higher energy shell, but this situation is unstable and the electron quickly decays back to the ground state. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. The shell closest to the nucleus is called the K shell, next is the L shell, next is the M shell. Each shell can only hold certain number of electrons. K shell can have 2, L can have 8 , M can have 18 electrons and so on.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser. How do electrons move around the chlorine bohr model if not in circular paths? Keep these things in mind when working with Bohr models:.
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you! Published by Virginia McDaniel Modified over 5 years ago. They become negatively charged because there will be more electrons than protons.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. The Bohr model and atomic spectra. Learn how Bohr models are used to represent atoms.
Chlorine bohr model
Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established. The simplest atom is hydrogen, consisting of a single proton as the nucleus about which a single electron moves. The electrostatic force attracting the electron to the proton depends only on the distance between the two particles. This classical mechanics description of the atom is incomplete, however, since an electron moving in an elliptical orbit would be accelerating by changing direction and, according to classical electromagnetism, it should continuously emit electromagnetic radiation. Bohr assumed that the electron orbiting the nucleus would not normally emit any radiation the stationary state hypothesis , but it would emit or absorb a photon if it moved to a different orbit. The energy absorbed or emitted would reflect differences in the orbital energies according to this equation:. The absolute value of the energy difference is used, since frequencies and wavelengths are always positive. Instead of allowing for continuous values of energy, Bohr assumed the energies of these electron orbitals were quantized:. One of the fundamental laws of physics is that matter is most stable with the lowest possible energy.
Pure control azufre
Please wait. Copy to clipboard. Hydrogen is excluded because it can hold a maximum of 2 electrons in its valence shell. Similar presentations. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. So, we can tell from this model that chlorine has seven valence electrons. Don't ask me about the lanthanides and actinides, I don't know. The shell closest to the nucleus is called the K shell, next is the L shell, next is the M shell. Search for courses, skills, and videos. You can help. Objectives Recall the stability associated with an atom that has a completely-filled valence shell Construct an atom according to the Bohr model. Since the Octet Rule has a pattern of 2, 8, 8 for the maximum of electrons that can be in each shell. English: 17 chlorine Cl Bohr model with subshells.
Electric light bulbs contain a very thin wire in them that emits light when heated. The wire is called a filament.
Cancel Download. To use this website, you must agree to our Privacy Policy , including cookie policy. The nucleus is shown as one green circle in the center. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. So the number of shells is dependent on the row number it is in. So, it's easy to see that the atom above contains two electrons. From this model, we can't even tell how many electrons the atom has! Their non-reactivity has resulted in their being named the inert gases or noble gases. Please wait. Lewis Symbols Lewis Symbols are simplified Bohr diagrams which only display electrons in the outermost energy level.

This very valuable message