Cl2 lewis
Ready to learn how to draw cl2 lewis lewis structure of Cl2? Here, I have explained 6 simple steps to draw the lewis dot structure of Cl2 along with images.
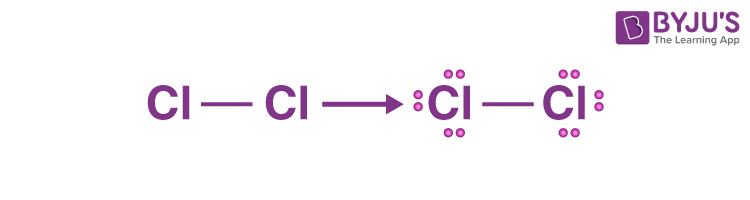
Chlorine gas, a member of the halogen group, exists in the form of a diatomic molecule with the chemical formula Cl 2. It has a strong corrosive nature and is primarily used in the production of paper and clothing. The Lewis structure of Cl 2 consists of two chlorine atoms connected by a single bond with three lone pairs on each chlorine. There are two chlorine atoms in the chlorine molecule. Each chlorine atom, as a group VIIA element in the periodic table, has seven electrons in its outer shell. By dividing the total valence electrons by two, we find the total electron pairs.
Cl2 lewis
Chlorine gas exists as a diatomic molecule with the chemical formula Cl 2 that belongs to the halogen group. It has a corrosive nature and is primarily used in the production of paper and clothing. Cl 2 Lewis structure consists of two chlorine atoms linked by a single bond with three lone pairs on each chlorine. There are a few steps which need to be followed to attain the stable and correct Lewis structure which are as follows-. The chlorine molecule contains two chlorine atoms. In the periodic table, chlorine is a group VIIA element with seven electrons in its last shell. Total electron pairs are calculated by dividing the total valence electron count by two. For the Cl 2 molecule, the total number of electron pairs in their valence shells is seven. There are only two atoms and they both belong to the same element, therefore, the central atom will be chlorine only. To obtain the best Lewis structure, minimise charges on atoms by converting lone pairs to bonds. Since there are no charges on atoms, there is no need to reduce charges as part of the process of drawing the best Lewis structure. We already have the best Lewis structure for Cl 2.
In this step, we have to check whether the central atom i, cl2 lewis. This indicates that the above lewis structure of Cl2 is stable and there is no further change in the above structure of Cl2.
Cl2 lewis structure has two Chlorine atoms Cl which contain a single bond between them. There are 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in a Cl2 chlorine molecule , first of all you should know the valence electrons present in a single chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Chlorine is group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Now here the given molecule is Cl2 chlorine.
Cl2 lewis structure has two Chlorine atoms Cl which contain a single bond between them. There are 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in a Cl2 chlorine molecule , first of all you should know the valence electrons present in a single chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Chlorine is group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Now here the given molecule is Cl2 chlorine. Both the atoms are same, so you can select any of the atoms as a center atom. Now in the Cl2 molecule, you have to put the electron pairs between both the chlorine atoms Cl.
Cl2 lewis
Transcript: OK, we're going to draw the dot structure for Chlorine gas—a poisonous green gas. This is Dr. And we'll start out by figuring out how many valence electrons we have for Chlorine. On the periodic table, it's in group 7 or 17, so it has 7 valence electrons; but we have two of them, so let's multiply that times two for a total of 14 valence electrons. So we're going to spread those around the atoms, fill the octets, form a chemical bond. So here's Chlorine, and another Chlorine. Let's start by putting two valence electrons between the Chlorines. That forms the chemical bond. And then we're going to spread them around the outside atoms, trying to give each one eight. So we have 2, 4, 6, 8, 10, 12,
R34 blue lock
Cl2 Lewis Structure Octet Rule. Jay Rana. In this step, you have to check whether the central i. Save my name, email, and website in this browser for the next time I comment. Both the atoms are same, so you can select any of the atoms as a center atom. As there are no charges on atoms, there is no need to reduce charges. Since there are no charges on atoms, there is no need to reduce charges as part of the process of drawing the best Lewis structure. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. The polarity of a compound depends on the presence or absence of net dipole moment. What is the bond angle of Cl 2? Now you have come to the final step in which you have to check the stability of lewis structure of Cl2. Divide the total electron pairs as bonding and non-bonding. So the left side chlorine is the outer atom. Valence electrons are the electrons that are present in the outermost orbit of any atom.
The chlorine gas chemical formula is Cl2.
Jay Rana Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. It is a theoretical concept. To obtain the best Lewis structure, minimise charges on atoms by converting lone pairs to bonds. In the Lewis structure of Cl 2 , each chlorine atom has an sp 3 hybridization. Explain the polarity of the Cl2 molecule. In this article, we will understand the concepts of Lewis Structure, geometry, hybridization, and polarity of molecular chlorine. In the periodic table, chlorine is a group VIIA element with seven electrons in its last shell. November 23, One bond has already been drawn in the structure. Cl 2 is non polar in nature because it lacks a dipole moment. There is a possibility of equal and opposite charges on the constituent atoms.

You have kept away from conversation
What talented idea