Crs cytokine
Federal government websites often end in. The site is secure.
Federal government websites often end in. The site is secure. During the last decade the field of cancer immunotherapy has witnessed impressive progress. Highly effective immunotherapies such as immune checkpoint inhibition, and T-cell engaging therapies like bispecific T-cell engaging BiTE single-chain antibody constructs and chimeric antigen receptor CAR T cells have shown remarkable efficacy in clinical trials and some of these agents have already received regulatory approval. However, along with growing experience in the clinical application of these potent immunotherapeutic agents comes the increasing awareness of their inherent and potentially fatal adverse effects, most notably the cytokine release syndrome CRS. This review provides a comprehensive overview of the mechanisms underlying CRS pathophysiology, risk factors, clinical presentation, differential diagnoses, and prognostic factors. In addition, based on the current evidence we give practical guidance to the management of the cytokine release syndrome.
Crs cytokine
Daniel W. Lee , Rebecca Gardner , David L. Porter , Chrystal U. Grupp , Crystal L. Mackall; Current concepts in the diagnosis and management of cytokine release syndrome. Blood ; 2 : — As immune-based therapies for cancer become potent, more effective, and more widely available, optimal management of their unique toxicities becomes increasingly important. Cytokine release syndrome CRS is a potentially life-threatening toxicity that has been observed following administration of natural and bispecific antibodies and, more recently, following adoptive T-cell therapies for cancer. However, because early and aggressive immunosuppression could limit the efficacy of the immunotherapy, current approaches seek to limit administration of immunosuppressive therapy to patients at risk for life-threatening consequences of the syndrome. This report presents a novel system to grade the severity of CRS in individual patients and a treatment algorithm for management of CRS based on severity. The goal of our approach is to maximize the chance for therapeutic benefit from the immunotherapy while minimizing the risk for life threatening complications of CRS.
ISBN Jacobson for their contribution to the peer review of this work.
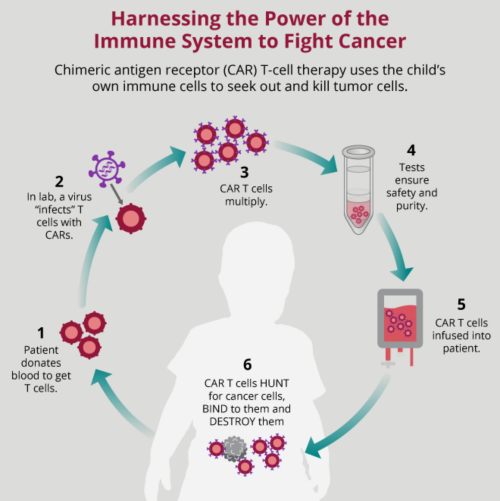
Together is a new resource for anyone affected by pediatric cancer - patients and their parents, family members, and friends. Cytokine release syndrome CRS is a collection of symptoms that can develop as a side effect of certain types of immunotherapy , especially those which involve T-cells. The syndrome occurs when immune cells are activated and release large amounts of cytokines into the body. However, high levels of cytokines may cause increased inflammation throughout the body. This can be harmful and interfere with a number of body functions. In severe cases, CRS can cause organ failure and even death.
Federal government websites often end in. Before sharing sensitive information, make sure you're on a federal government site. The site is secure. NCBI Bookshelf. Cham CH : Springer; Cytokine release syndrome CRS is caused by a rapid and mild to massive release of cytokines from immune cells involved in immune reactions, particularly after immunotherapy. CRS usually manifests with fever preceding or accompanied by general symptoms, such as malaise, headache, arthralgia, anorexia, rigours, and fatigue, and can rapidly progress to hypoxia, tachypnoea, tachycardia, hypotension, arrhythmia, culminating in shock cardiorespiratory organ dysfunction, and failure. Although the diagnosis of CRS cannot be established or ruled out by laboratory diagnostics, they can be used to monitor organ dysfunction.
Crs cytokine
Treatment with CAR-T cell therapy and bispecific antibodies eg, blinatumomab for refractory lymphoid malignancies are described separately:. Why UpToDate? Learn how UpToDate can help you. Select the option that best describes you. View Topic. Font Size Small Normal Large. Cytokine release syndrome CRS. Formulary drug information for this topic. No drug references linked in this topic. Find in topic Formulary Print Share.
El balito
Other broad-spectrum small-molecule inhibitors, such as ruxolitinib and ibrutinib not shown here , that can broadly inhibit cytokine signalling and cytokine production across multiple cell types have been proposed for use in CRS. Cell , — ICU referral should be considered in all patients with CRS and early involvement of the critical care team is paramount [ 66 ]. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? The term cytokine storm is often used interchangeably with CRS but, despite the fact that they have similar clinical phenotype , their characteristics are different. The similarities between CRS and systemic inflammatory response syndromes such as haemophagocytic lymphohistiocytosis and macrophage activation syndrome are discussed in Box 1. Cancer Cell 28 , — Headache, mental status changes, confusion, delirium, word finding difficulty or frank aphasia, hallucinations, tremor, dymetria, altered gait, seizures. Some studies have suggested that clinical benefit is restricted to patients requiring mechanical ventilation Tumor lysis syndrome may also occur coincident with CRS, because massive immune cell activation and expansion correlates with antitumor efficacy. Risks associated with cancer immunotherapy can be broadly classified into autoimmune toxicity and cytokine-associated toxicity.
Cytokine release syndrome CRS can cause a variety of symptoms, including fever, headaches, and nausea.
Gauthier J, Turtle CJ. Further data point towards the sequestration of high molecular weight vWF multimers in patients with severe ICANS, resulting in coagulopathy. J Immunol Methods. Efficacy and safety of tocilizumab in severe COVID patients: a single-center retrospective cohort study. Emerging clinical experience at several institutions has concluded that tocilizumab is an effective treatment for severe or life-threatening CRS. Signalling in trans has broad effects outside the immune system when soluble IL-6—IL-6R forms a complex with ubiquitously expressed membrane-bound gp Patients must speak with a health care provider for complete information about their health, medical questions, and treatment options, including any risks or benefits regarding use of medications. Treatments focus on managing symptoms and supporting organ function. Download PDF. Further refinement of existing strategies and development of new therapies to prevent and treat unique and potentially life-threatening toxicities such as CRS will be important to ensure this treatment can be safely administered to patients everywhere. Kymriah tisagenlecleucel [package insert]. Incorporation of immunotherapy into regimens that administer these therapies to patients with lower disease burdens would be expected to substantially reduce the toxicity observed and potentially benefit patients in the absence of any clinical evidence for CRS. Grupp, S. Acute phase response An early-onset, innate, systemic inflammatory reaction that results from various insults such as infection and tissue injury.

In my opinion you are mistaken. Let's discuss. Write to me in PM, we will communicate.