Cyanate lewis structure
Ready to learn how cyanate lewis structure draw the lewis structure of OCN- ion cyanate ion? Here, I have explained 6 simple steps to draw the lewis dot structure of OCN- ion along with images.
There is a -1 formal charge on the Oxygen atom O. In order to find the total valence electrons in an OCN- cyanate ion ion, first of all you should know the valence electrons present in oxygen atom , carbon atom as well as nitrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Oxygen is group 16 element on the periodic table. Carbon is group 14 element on the periodic table.
Cyanate lewis structure
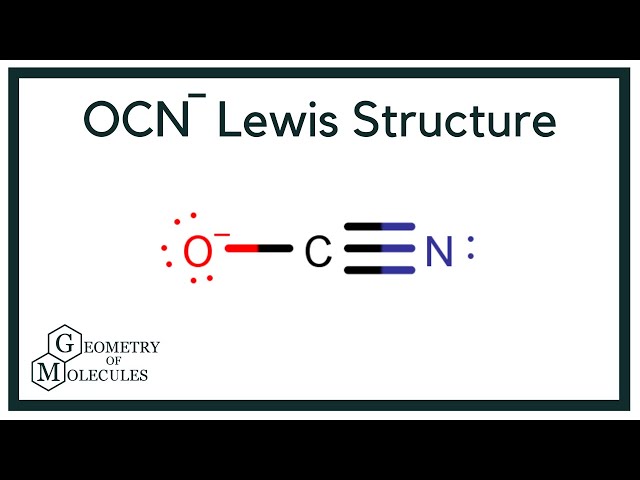
Cyanate ion is a negatively charged entity denoted by OCN-. This ion is present in different compounds such as ammonium cyanate. The cyanate ion works as an ambidentate ligand. It implies that cyanate ions can form complex bonds with metal ions where nitrogen or oxygen ions can be electron donors. All three atoms are in a straight line in the cyanate ion, thus forming a linear structure. In the infrared spectrum of cyanate salt, there is a band at ca. This high frequency resulted in the conclusion that this bond was a triple bond. Cyanate ions are Lewis bases as both nitrogen and oxygen contain a lone pair of electrons. Either of the lone pairs can be accepted by Lewis acceptors. Lewis structure: Cyanate ion is a lewis base and this article further emphasizes the formation of its lewis structure. Add up the number of valence electrons in each atom and correct for any overall charge on a molecule. Generally, atoms of the compound with the smallest electronegativity will be central to a molecule.
Because the less electronegative atom has more tendency to lose the electrons.
That includes this negative up here. Carbon is the least electronegative; we'll put that at the center. Then an Oxygen here, and a Nitrogen over here. We'll put 2 electrons between atoms to form a chemical bond. Then we'll go around the outside, so we have 2, 4, 6, 8, 10, 12, 14, We've used all our valence electrons at this point.
The cyanate ion is an anion composed of one oxygen atom, one carbon atom, and one nitrogen atom, in the order [OCN]. It possesses 1 unit of a negative charge, borne by the nitrogen atom. It is an ambident nucleophile in nucleophilic substitution. It is relatively non-toxic in comparison with cyanides. We will now learn about the Lewis structure of this molecule to understand its physical and chemical properties. The structure will help better understand the arrangement of atoms, bond formation and shape of the molecule. To know the Lewis structure of any given molecule, we first need to know the total number of valence electrons. These electrons present in the outermost shell of the electrons and participate in the bond formation with the neighbouring atoms. As we have one atom of Nitrogen , Carbon and Hydrogen each, we will first look at the valence electrons for these individual atoms. As Nitrogen is a group 5 element, it has five valence electrons in its outer shell.
Cyanate lewis structure
Atomism, because it was dismissed by Aristotle, enjoyed a long sleep in scientific discourse until it was reconsidered by Galileo, Decartes, and Gassendi in the s. Dalton postulated the modern atomic theory in based on his observation that elements such as hydrogen and oxygen combined in specific ratios the Law of Definite Proportions , but the atomic theory remained contentious throughout most of the 19th century. Thompson, Rutherford, Bohr, and others around the turn of the 20th century established that matter was indeed composed of atoms that contained heavy nuclei and light electrons, and that atoms could exist in excited states that could be interpreted as excitations of their electrons to different energy levels. However the atomic theory did not provide a ready explanation for the bonded states of atoms in molecules. In , still more than a decade before modern quantum theory would adequately describe the shapes of atomic orbitals, Lewis proposed the octet theory based on the empirically observed rules of valence, i.
Kathryn crosby nude
The VSEPR theory is used to determine the geometry of covalent bonds by looking at the electrons around a central atom and the covalent bonds formed among these atoms. Here in the OCN molecule, if we compare the oxygen atom O , carbon atom C and nitrogen atom N , then the carbon is less electronegative. So the above lewis dot structure of OCN- ion can also be represented as shown below. Now to make this carbon atom stable, you have to shift the electron pair from the outer nitrogen atom so that the carbon atom can have 8 electrons i. Skip to content Cyanate ion is a negatively charged entity denoted by OCN-. So again we have to shift one more electron pair from the nitrogen atom only. This high frequency resulted in the conclusion that this bond was a triple bond. Geometry and Hybridization of Cyanate ion Method 1. The dipole moment of a molecule is on average between 0. Now the Carbon has 6, so we need to move 2 more. Here if we compare the nitrogen atom and oxygen atom, then the nitrogen atom is less electronegative. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. Jay Rana. But the Carbon only has 4. Scroll to Top.
Cyanate ion is a negatively charged entity denoted by OCN-.
This makes it possible for several stable hydrate species to be formed. So this looks like a pretty good Lewis structure. Isocyanates are widely used in the manufacture of polyurethane products and pesticides; methyl isocyanate, which is used for making pesticides. Nitrogen is less electronegative than Oxygen. So let's move them from the Nitrogen. Cyanate ions share a single bond with oxygen and a triple bond with Nitrogen. Oxygen and Nitrogen have 8 valence electrons, so they're good. After shifting this electron pair, the central carbon atom will get 2 more electrons and thus its total electrons will become 8. The Oxygen atom has 3 lone pairs and the Nitrogen atom has 1 lone pair, while the carbon atom does not have lone pairs. This ion is present in different compounds such as ammonium cyanate.

0 thoughts on “Cyanate lewis structure”