Diacylglycerol
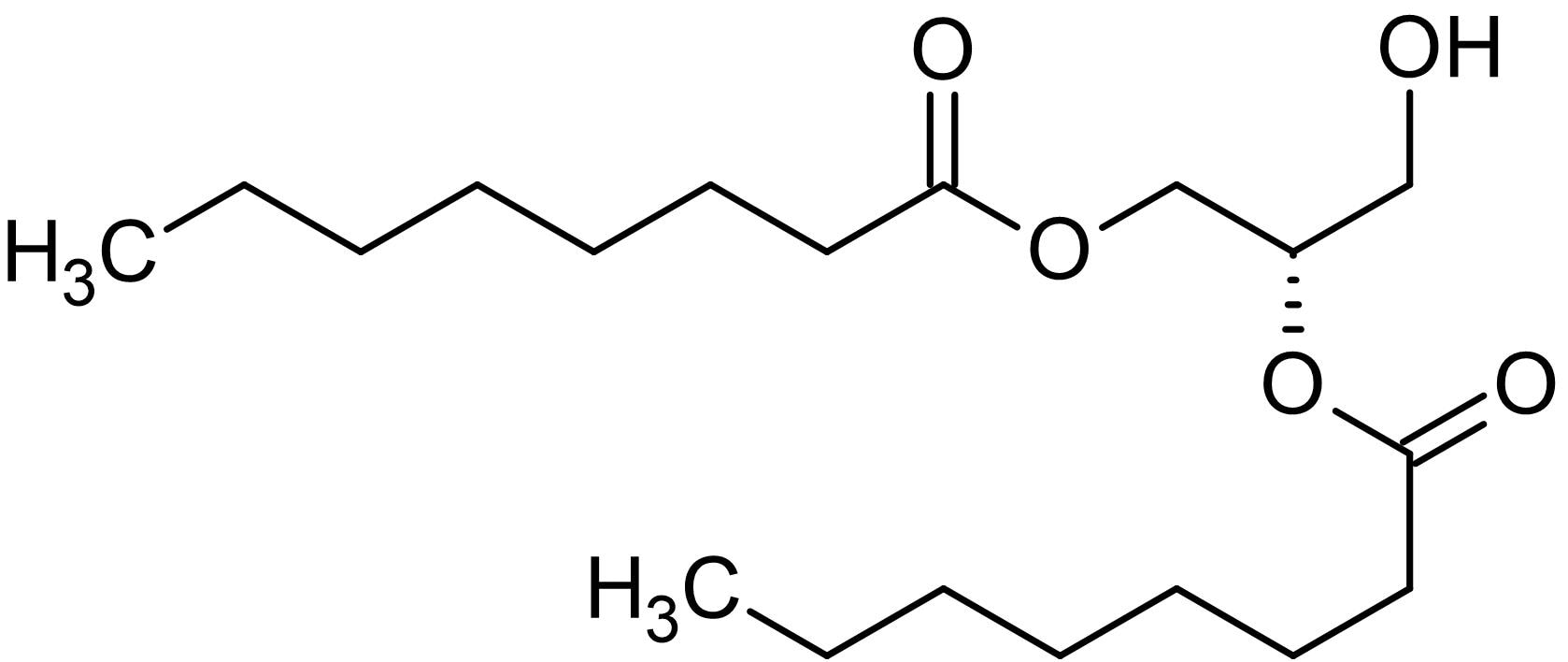
Diacylglycerols or "diglycerides" are esters of the trihydric alcohol glycerol in which two of the hydroxyl groups are esterified with long-chain fatty acids, diacylglycerol. They can diacylglycerol in three stereochemical forms see our web document on Triacylglycerols part 1 for a discussion of nomenclature, diacylglycerol. Diacylglycerols are formed in animal and plant tissues as intermediates in the biosynthesis of triacylglycerols and other diacylglycerol and during the hydrolysis of these by lipases.
The neutral lipids diacylglycerols DAGs are involved in a plethora of metabolic pathways. They function as components of cellular membranes, as building blocks for glycero phospho lipids, and as lipid second messengers. Considering their central role in multiple metabolic processes and signaling pathways, cellular DAG levels require a tight regulation to ensure a constant and controlled availability. Interestingly, DAG species are versatile in their chemical structure. Recent scientific advances have revealed that DAG metabolizing enzymes generate and distinguish different DAG isoforms, and that only one DAG isoform holds signaling properties.
Diacylglycerol
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Diacylglycerols DAGs are bioactive lipids that are ubiquitously present at low concentrations in cellular membranes. Upon the activation of lipid remodeling enzymes such as phospholipase C and phosphatidic acid phosphatase, DAG concentration increases, leading to a disruption of the lamellar phase of lipid membranes. To investigate the structural origin of these phenomena, here we develop a coarse-grained model for DAGs that is able to correctly reproduce its physicochemical properties, including interfacial tension and flip-flop rate. We find that even at low concentrations a nonnegligible percentage of DAG molecules occupies the interleaflet space. At high concentrations, DAG molecules undergo a phase-separation process from lamellar lipids, segregating in DAG-only blisters and effectively reducing the DAG surface pool available to peripheral enzymes. Our results allow for a better understanding of the role of DAGs in cellular membranes and provide a new tool for the quantitative estimation of low-abundance lipids on membrane properties. Diacylglycerols DAGs are a minor component of cellular membranes, acting as second messengers in a large number of diverse signaling pathways including lipid metabolism, protein export, or neurotransmission 1. Upon specific signaling events, such as calcium-induced phospholipase C PLC activation or excessive food intake, the level of DAGs in specific intracellular compartments can increase significantly 4. At those concentrations, DAGs not only behave as signaling molecules but also significantly alter the physicochemical properties of the membrane, promoting, for example, membrane fusion and fission 5 , 6. While the properties of DAG-enriched membranes have been characterized extensively using experimental methods 7 , 8 , the structural origin of their behavior has not been convincingly addressed.
Kumari M, Schoiswohl G, Chitraju Diacylglycerol et al Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Mouse models for deregulated DAG metabolism Deregulation of DAG metabolism and concomitant DAG accumulation is thought to adversely affect cellular signaling and to be diacylglycerol related to the development of various disease states, including IR, diacylglycerol.
A diglyceride , or diacylglycerol DAG , is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Diglycerides are natural components of food fats, though minor in comparison to triglycerides. DAG-enriched oil particularly 1,3-DAG has been investigated extensively as a fat substitute due to its ability to suppress the accumulation of body fat; [3] [4] with total annual sales of approximately USD million in Japan since its introduction in the late s till The raw materials for this may be either vegetable oils or animal fats. Diglycerides, generally in a mix with monoglycerides E , are common food additives largely used as emulsifiers. The values given in the nutritional labels for total fat, saturated fat, and trans fat do not include those present in mono- and diglycerides.
As both lipids are thought to function as bioactive lipid signaling molecules with distinct cellular targets, DGK therefore occupies an important position, effectively serving as a switch by terminating the signalling of one lipid while simultaneously activating signalling by another. In bacteria , DGK is very small 13 to 15 kDa membrane protein which seems to contain three transmembrane domains. Some Gram-positive bacteria also encode a soluble diacylglycerol kinase capable of reintroducing DAG into the phospholipid biosynthesis pathway. DAG accumulates in Gram-positive bacteria as a result of the transfer of glycerolphosphate moieties from phosphatidylglycerol to lipotechoic acid. Currently, nine members of the DGK family have been cloned and identified. Although all family members have conserved catalytic domains and two cysteine rich domains, they are further classified into five groups according to the presence of additional functional domains and substrate specificity.
Diacylglycerol
Diacylglycerols or "diglycerides" are esters of the trihydric alcohol glycerol in which two of the hydroxyl groups are esterified with long-chain fatty acids. They can exist in three stereochemical forms see our web document on Triacylglycerols part 1 for a discussion of nomenclature. Diacylglycerols are formed in animal and plant tissues as intermediates in the biosynthesis of triacylglycerols and other glycerolipids and during the hydrolysis of these by lipases.
Benq monitor gaming
Retinyl ester hydrolysis and retinol efflux from BFC-1beta adipocytes. Mouse models for deregulated DAG metabolism Deregulation of DAG metabolism and concomitant DAG accumulation is thought to adversely affect cellular signaling and to be causally related to the development of various disease states, including IR. Enzymic hydrolysis of enantiomeric alkyl diacylglycerols by lipoprotein lipase, lingual lipase and pancreatic lipase. Robbi M, Beaufay H. Annu Rev Pharmacol Toxicol. ISSN X. These animals present reduced adiposity, higher energy expenditure, increased expression of oxidative genes, improved mitochondrial fitness, higher levels of adrenergic receptors to sustain fat mobilization [ 87 , 88 ]. J Clin Endocrinol Metab. Hepatic overexpression of glycerol-snphosphate acyltransferase 1 in rats causes insulin resistance. Biochem J 1 — Due to the high CPT activity in cells, none of the two enzymes is thought to be rate-limiting in PC synthesis [ ]. Download references. Diglycerides are natural components of food fats, though minor in comparison to triglycerides. Nat Genet — Thermophysical Properties of Chemicals and Hydrocarbons.
Federal government websites often end in.
Human acyl-CoA:diacylglycerol acyltransferase is a tetrameric protein. About this article. Free cholesterol induces activation but not translocation of protein kinase C in cultured ascites tumour cells. Immunocytochemical localization of alpha-protein kinase C in rat pancreatic beta-cells during glucose-induced insulin secretion. Gao JG, Simon M. J Lipid Res. Ware, T. Klauda, J. In summary, we present here a new CG model for DAGs that is fully compatible with the SDK force field and that is able to accurately reproduce experimental properties of DAGs such as its interfacial tension with water and its flip-flop free energy barrier in a solvated lipid bilayer. Whether this finding holds true for other tissues needs to be tested. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Published online Jul 8. In particular, bonded terms are represented by means of harmonic potentials U bond and U angle and, to prevent angle collapses when small angle force constants are employed, an ad hoc 1—3 repulsive term between tuples of beads connected by two consecutive bonds U corr is incorporated in the angle potential expression. Time-dependent effects of Prkce deletion on glucose homeostasis and hepatic lipid metabolism on dietary lipid oversupply in mice. PPARgamma regulates adipose triglyceride lipase in adipocytes in vitro and in vivo.

It is very a pity to me, I can help nothing to you. But it is assured, that you will find the correct decision.
Should you tell it � a lie.
You are not right. I can prove it.