Difference between isothermal and adiabatic process class 11
As per the thermodynamic terminology, in the adiabatic process, there is no exchange of heat from the system to its surroundings neither during expansion nor during compression. Whereas in the isothermal process, the temperature remains constant throughout the work. This article will definitely help to understand the difference between isothermal and adiabatic process.
Isothermal and Adiabatic Processes are two different processes that are related to heat and energy in thermodynamics. Thermodynamics is the branch of physics which deals with the heat transfer and thermal properties of matter. In thermodynamics, the isothermal process is the process that occurs at constant temperature and the exchange of heat and energy takes place between the system and the surroundings. Adiabatic Process in thermodynamics stands for the process where a system is isolated from its surroundings and there is no heat and energy exchange between the system and the surroundings. In this article, we will learn briefly about the isothermal and adiabatic processes, with a major focus on the difference between isothermal and adiabatic processes.
Difference between isothermal and adiabatic process class 11
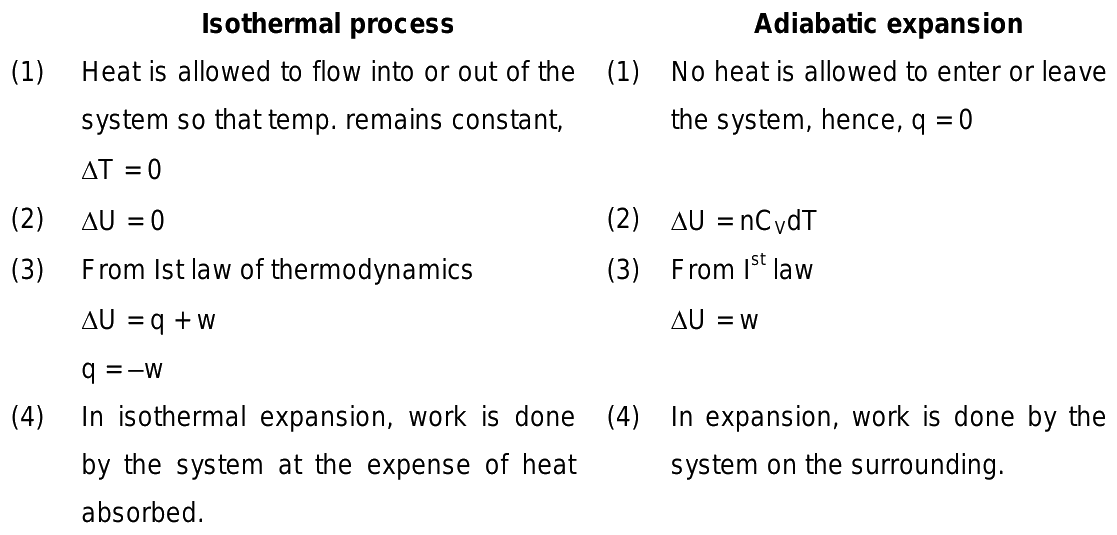
The difference between isothermal and adiabatic processes has to be comprehended to understand their industrial applications. Both these processes are more frequently discussed in thermodynamics. Both these processes are entirely opposite to each other. The major difference between these two types of processes is that in the adiabatic process, there is no transfer of heat towards or from the liquid. On the other hand, in the isothermal process , there is a transfer of heat to the surroundings to make the overall temperature constant. These are some differences between the isothermal and adiabatic processes. Put your understanding of this concept to test by answering a few MCQs. Your Mobile number and Email id will not be published. Post My Comment. Test your knowledge on Isothermal and adiabatic processes differences Q 5. Start Quiz. Your result is as below. Login To View Results. Did not receive OTP?
Work done in an Adiabatic reversible compression is given as. More Articles for Physics.
Thermodynamics deals with the two most important concepts of thermal physics, i. The adiabatic process is the one that deals with the transfer of energy between the system and surroundings in the form of work. In an adiabatic process, there is no transfer of any heat or mass between the system and the surroundings. The main concept behind the difference between adiabatic and isothermal processes is that while the former is isolated from the surroundings, the latter is not. Unlike adiabatic processes, the isothermal process is the one in which there is heat transfer between the system and the surroundings and the temperature of the system remains constant throughout the process. The temperature is kept constant by the transfer of heat between the system and the surroundings.
The difference between isothermal and adiabatic processes has to be comprehended to understand their industrial applications. Both these processes are more frequently discussed in thermodynamics. Both these processes are entirely opposite to each other. The major difference between these two types of processes is that in the adiabatic process, there is no transfer of heat towards or from the liquid. On the other hand, in the isothermal process , there is a transfer of heat to the surroundings to make the overall temperature constant. These are some differences between the isothermal and adiabatic processes. Put your understanding of this concept to test by answering a few MCQs. Your Mobile number and Email id will not be published. Post My Comment.
Difference between isothermal and adiabatic process class 11
We can now comprehend the difference between adiabatic and isothermal :. The behavior of a thermodynamic system and its relationship to temperature changes are described by the ideas of the isothermal process, isochoric process, isobaric process, and adiabatic process in thermodynamics. JEE Main. Topic Name:. Difference Between Isothermal and Adiabatic Process.
Assoholics.cc
The temperature varies because of the internal system changes. Generally, there are two conditions under which the isothermal process can work:. Engineering Exam Experiences. Your Mobile number and Email id will not be published. Process of sound propagation in air is an example of an adiabatic process. Customize your course in 30 seconds Start Learning Now. The isothermal process has a slower transformation flow. Also, reach out to the test series available to examine your knowledge regarding related exams. This is especially true in cases of irreversible processes. Now learn Live with India's best teachers. The transformation is fast in such a process. Adiabatic process describes a process that remains aloof from its surroundings. Photoelectric Effect Experiment. It is defined as one of the thermodynamic processes which occur at a constant temperature. The pressure is less at a given volume.
Thermodynamics uses the concepts isothermal process and adiabatic process to explain the behavior of a thermodynamic system and its relation to the temperature changes. Isothermal process is a process that happens under constant temperature, but other parameters regarding the system can be changed accordingly.
The graph of adiabatic process is shown below. Hire With Us. Adiabatic process is a type of thermodynamic process in which where there is no heat transfer. It is vitally used as a model to show real process models in engineering and also for major comparisons between the systems. The main concept behind the difference between adiabatic and isothermal processes is that while the former is isolated from the surroundings, the latter is not. Solution: It is possible by the work done on the system and so, the temperature of the system will change even if there is no heat content added or removed from it. While putting the ice into the icebox, no heat will go out and no heat will come in. This happens when the surrounding temperature T is less than the temperature of the system T S i. No heat goes inside or leaves the system. Improve Improve. The temperature of the surroundings changes irrespective of changes in the internal temperature of the refrigerator. It is defined as one of the thermodynamic processes which occur at a constant temperature. The isothermal process is the thermodynamic process in which there is no change in the temperature throughout the process, i. Adiabatic Process Adiabatic process is a type of thermodynamic process in which where there is no heat transfer.

Excuse for that I interfere � here recently. But this theme is very close to me. Write in PM.
Yes, really. So happens.