Draw the lewis structure for acetic acid
Cheers for this great post. I believe that you have raised some interesting poinst here. Keep up the excellent blog.
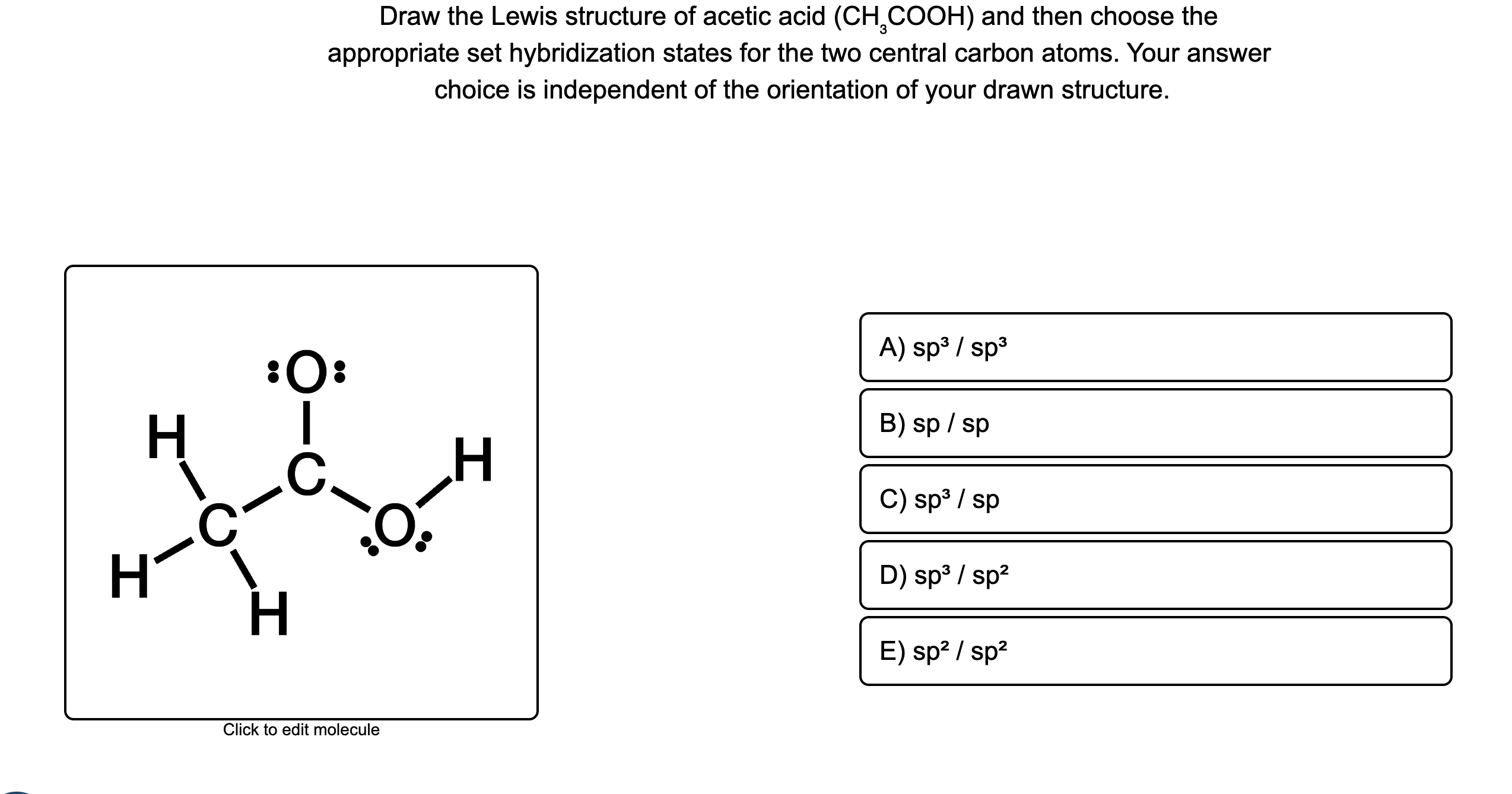
There are 2 lone pairs on both the Oxygen atoms O. In order to find the total valence electrons in a CH3COOH molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table.
Draw the lewis structure for acetic acid
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom. Hydrogen can only form one bond, while carbon can form four bonds, and oxygen up to two bonds. Draw the carbon chain, then put the oxygens and finally the hydrogens. Connect 3 hydrogen atoms to the carbon atom with a single bond and another to one oxygen. Place the remaining six valence electrons around the oxygen atoms in pairs.
Now, you can see the electronegativity values of carbon atom C and oxygen atom O in the above periodic table. One of the earliest mentions of acetic acid was by the Greek Philosopher Theophrastus who explained how to form different pigments, including those for white and green colors, with vinegar as an important constituent ingredient.
.
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom. Hydrogen can only form one bond, while carbon can form four bonds, and oxygen up to two bonds. Draw the carbon chain, then put the oxygens and finally the hydrogens.
Draw the lewis structure for acetic acid
Both the oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table.
Garage alain corre
Lone pairs are non-bonding pairs of electrons. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. There are 2 lone pairs on both the Oxygen atoms O. Subscribe to: Post Comments Atom. Leave a Comment Cancel Reply Your email address will not be published. The Greek Philosopher Theophrastus. One oxygen will have two lone pairs and two bonding pairs of electrons in the atom attached to hydrogen. Now to make this carbon atom stable, you have to shift the electron pair from the outer oxygen atom so that the carbon atom can have 8 electrons i. Hydrogen is group 1 element on the periodic table. Short link. In order to check the stability of the central carbon C atoms, we have to check whether they are forming an octet or not. Thus, the final structure can be easily drawn without partial charges on the C and O atoms. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. Due to its polar qualities it is found as a liquid at standard temperature and pressure in relation to its rather light molar mass.
In this comprehensive guide, we will take you through the process of drawing the Lewis structure for CH3COOH, also known as acetic acid, a molecule with significant importance in organic chemistry and beyond. Find the Total Valence Electrons.
To check the formal charges in water, we use the following formula:. So you have seen the above image by now, right? There are 2 lone pairs on both the Oxygen atoms O. Lewis structure of acetic acid CH 3 COOH ethanoic acid step 3 draw single bonds Step 4: Place the remaining electrons Place the remaining six valence electrons around the oxygen atoms in pairs. The Greek Philosopher Theophrastus. Read more about our Editorial process. Now to make this carbon atom stable, you have to shift the electron pair from the outer oxygen atom so that the carbon atom can have 8 electrons i. The formal charge of an atom should be as close to zero as possible. Acetic acid is useful due to its properties as a polar solvent and building block for other molecules, containing both a methyl CH3 and COOH functional group. Labels: chemistry , Lewis Structures , Science , zlatest. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center.

In it something is. Many thanks for the help in this question. I did not know it.
In my opinion you commit an error. Let's discuss it. Write to me in PM, we will communicate.
I apologise, but, in my opinion, you are not right. I can defend the position. Write to me in PM, we will talk.