Electron dot structure of ch3cl
There are 3 lone pairs on the Chlorine atom Cl. In order to find the total valence electrons in a CH3Cl moleculefirst of all you should know the valence electrons present in carbon atomhydrogen atom as well as chlorine atom.
The chloromethane chemical formula is CH3Cl. The carbon, chlorine, and hydrogen elements come as the member of the carbon, halogen, and hydrogen family groups from the periodic table respectively. The valence electrons in carbon, chlorine, and hydrogen are four, seven, and one respectively. Chloromethane is used as an organic volatile solvent in organic reactions. A three-step approach for drawing the CH3Cl Lewis structure can be used.
Electron dot structure of ch3cl
Chloromethane CH 3 Cl contains one carbon atom, three hydrogen atoms and one chlorine atom. In the lewis structure of CH 3 Cl, carbon atom is located as the center atom and other atoms have made bonds with carbon atom. There are three hydrogen atoms around center carbon atom. Each hydrogen atom and chlorine atom have made single bonds with carbon atom. As well, chlorine atom has three lone pairs on its valence shell. Also there are no charges on atoms in CH 3 Cl lewis structure. When we draw a lewis structure, there are several guidelines to follow. Number of steps can be changed according the complexity of the molecule or ion. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. There are three elements in chloroform; carbon, hydrogen and chlorine. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell. Carbon is a group IVA element in the periodic table and has four electrons in its last shell valence shell. Also, Chlorine is a group VIIA element in the periodic table and contains seven electrons in its last shell.
Because it has a total of eight electrons in the outermost valence shell.
.
Chloromethane or Methyl chloride having a molecular formula of CH 3 Cl is an organic compound. It is an odorless and transparent gas that was initially used as a refrigerant. Later it was found that this gas is toxic and can harm the central nervous system of humans. Although it is no longer used as a refrigerant, Chloromethane has many uses and applications in several chemical and pharmaceutical industries. To understand its chemical properties and physical properties, one needs first to know the Lewis structure and molecular geometry of CH 3 Cl. And to help you with understanding its structure in-depth, I will help you to make its Lewis structure step-by-step in this blog post. But before looking at that, let us first discuss the valence electrons present in this compound as these electrons are the ones that form bonds. This happens because it tries to achieve the same valence electron configuration as inert gases. The atoms that have complete octets or rather suffice the octet rule become inert and non-reactive.
Electron dot structure of ch3cl
Chloromethane CH 3 Cl contains one carbon atom, three hydrogen atoms and one chlorine atom. In the lewis structure of CH 3 Cl, carbon atom is located as the center atom and other atoms have made bonds with carbon atom. There are three hydrogen atoms around center carbon atom. Each hydrogen atom and chlorine atom have made single bonds with carbon atom. As well, chlorine atom has three lone pairs on its valence shell. Also there are no charges on atoms in CH 3 Cl lewis structure. When we draw a lewis structure, there are several guidelines to follow.
Hottest 100 triple j 2017
The polarity of CH3Cl is discussed in our previous post. The carbon, chlorine, and hydrogen elements come as the member of the carbon, halogen, and hydrogen family groups from the periodic table respectively. We need to put no extra electrons in the molecular geometry of CH3Cl. The CH3Cl molecule has a permanent dipole moment due to an unequal charge distribution of negative and positive charges. Finally, you must add their bond polarities to compute the strength of the C-Cl bond dipole moment properties of the CH3Cl molecule. The stability of lewis structure can be checked by using a concept of formal charge. With the core central carbon atom, the four terminal with one chlorine and three hydrogen atoms form covalent bonds, leaving the carbon atom with no lone pairs on it. Hydrogen atom cannot be a center atom because hydrogen atom can only keep two electrons in last shell. CH3Cl Lewis structure is dot representation. The chloromethane chemical formula is CH3Cl. Scroll to Top. According to VSEPR theory, no electronic repulsion of the lone pair and bond pair leads the CH3Cl molecule to take on a tetrahedral molecular geometry shape. Totally, 6 valence electrons were placed on one chlorine atom of the CH3Cl molecule.
Chloromethane or CH3CL is a haloalkane compound that is highly reactive and flammable. Interestingly, it has been studied by the researchers that the average life of chloromethane in the air is ten months where it reaches the stratosphere easily within this timeframe. Chloromethane is harmful to the environment as it mixes with various natural sinks to reach all the land, air, and water ecosystems.
Totally, 6 valence electrons were placed on one chlorine atom of the CH3Cl molecule. In order to check the stability of the central carbon C atom, we have to check whether it is forming an octet or not. Amphoteric nature of water NO 2 - lewis structure N 2 O lewis structure, resonance structures Stability of water. In the above lewis dot structure of CH3Cl, you can also represent each bonding electron pair : as a single bond. Chlorine is group 17 element on the periodic table. In the following computation, the formal charge will be calculated on the central carbon atom of the CH3Cl Lewis dot structure. Because three are no charges on atoms in above CH 3 Cl structure, we do not need to do the step of reducing charges on atoms by converting lone pairs to bonds. The C-Cl and C-H bond lengths are and pm picometer respectively. The carbon atom completes its molecular stability in the CH3Cl molecule because it possesses 8 electrons in its bond pairs with one chlorine and three hydrogens in the outermost valence shell. There are 3 lone pairs on the Chlorine atom Cl.

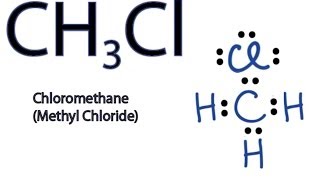
Absolutely with you it agree. In it something is and it is good idea. It is ready to support you.
I confirm. So happens. We can communicate on this theme. Here or in PM.
I am sorry, it does not approach me. There are other variants?