Electron dot structure of hno3
Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents Lewis Electron Dot Structures. Nitric acid is a strong oxidizing agent and it dissolves practically all metals except gold and platinum and some other precious metals. As such, electron dot structure of hno3, is an important raw material for the chemical and pharmaceutical industry.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
Electron dot structure of hno3
Draw the Lewis structure of HCN. Draw a Lewis structure of nitric oxide, NO. Draw the Lewis structure of B e C l 2. Draw the Lewis structure for S F 6. Draw the structure of : Perchloric acid. In the Lewis structure of acetic acid, there are. Draw the Lewis structure of iodine pentafluoride, I F 5. Draw the structure of an amino acid. Draw the structure of phosphinic acid H 3 P O 2. Draw a Lewis structure for nitrogen trichloride, N C l 3.
Reaction Mechanism. Draw the Lewis structure for S F 6. Third Law of Thermodynamics.
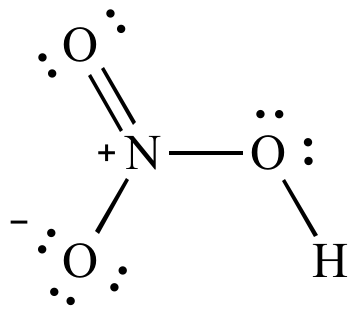
HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element. There are no lone pairs on nitrogen atom and also there are charges on one oxygen atom and nitrogen atom. You can see those signs in the following figure. There are some steps to follow to draw the HNO 3 lewis structure and those steps are explained in detail in this tutorial. Important: Drawing correct lewis structure is important to draw resonance structures correctly. Hydrogen is a group IA element and has one electron in its last shell.
The nitrogen atom is at the center and it is surrounded by 2 oxygen atoms and one O-H bond. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of HNO3. Here, the given molecule is HNO3. In order to draw the lewis structure of HNO3, first of all you have to find the total number of valence electrons present in the HNO3 molecule.
Electron dot structure of hno3
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs.
307 pounds in kg
Which one of the following molecules contains no pi - bond? Periodic Trend: Atomic Radius. Solubility Rules. Which one of the following pairs consists of only paramagnetic species. Step 1 : The central atom will be the N atom since it is the less electronegative H is a terminal atom — it cannot be a central atom. Production of Hydrogen. Born Haber Cycle. Periodic Table Charges Review. A coordinate bond is a dative bond. Amphoteric Species. Alkane Reactions. Chemical Thermodynamics 1h 48m. The Electron Configuration Review.
Nitric acid HNO3 , a highly corrosive acid, is a very important chemical. It is usually a colorless liquid, but the older samples turn pale yellow because it gets decomposed into water and oxides of nitrogen.
Hydrogen is a group IA element and has one electron in its last shell. Millikan Oil Drop Experiment. Related Practice. Intensive vs. Naming Alkanes. Calculate Oxidation Numbers. Orientations of D Orbitals. Naming Ionic Compounds. Intermolecular Forces and Physical Properties. McGoran, J. Draw a Lewis structure of nitric oxide, NO. Periodic Trend: Successive Ionization Energies.

I apologise, but, in my opinion, you are not right. Write to me in PM, we will talk.
I apologise, but, in my opinion, you commit an error. Write to me in PM, we will communicate.