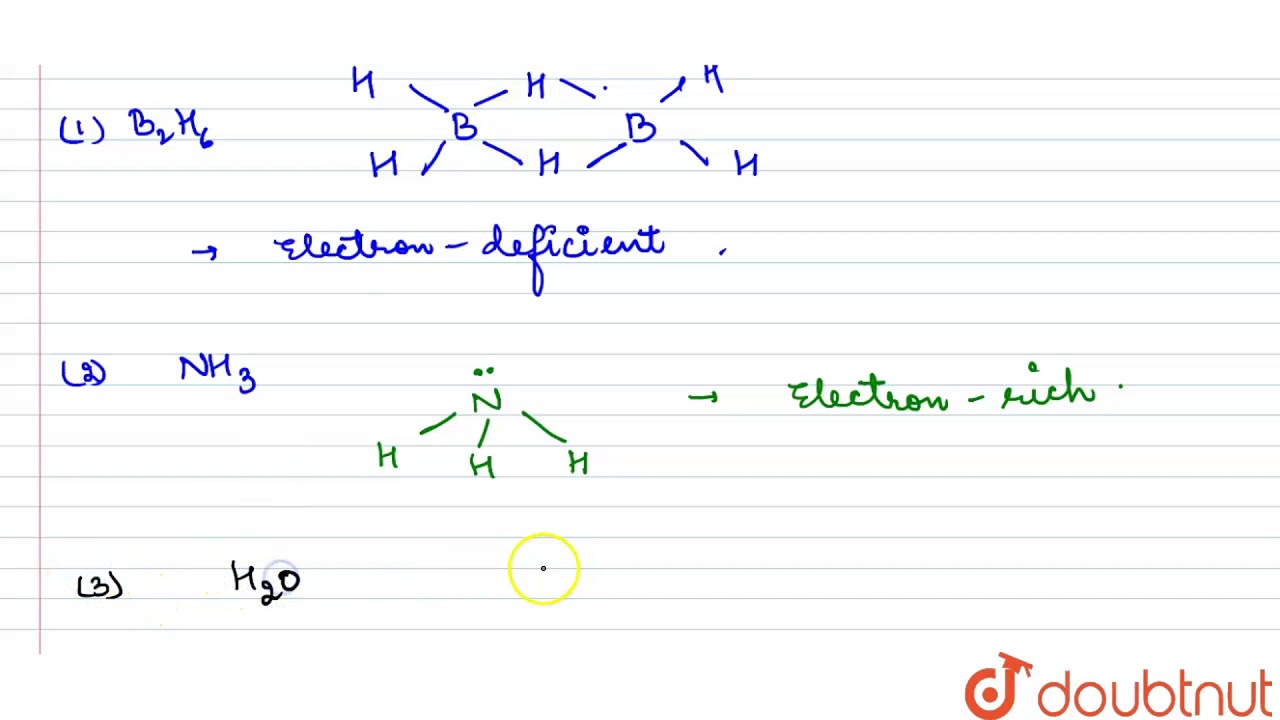
Electron rich hydrides
N H 3 is electron rich hydride. Describe electron-rich hydride. Which of the following hydrides is an electron-rich hydride?
Define the following terms: a electron-deficient b electron - precise c electron-rich compound. Justify your answer with an example? Example: CH 4 , SiH 4 , etc. What do you understand by i electron-deficient, ii electron-precise, and iii electron-rich compounds of hydrogen? Provide justification with suitable examples. Byju's Answer.
Electron rich hydrides
Hydrides - Hydrides are the binary compound of hydrogen with other elements. Let 'X" be any element, then -. Ex - MgH 2 is the hydride of Magnesium. Based upon the number of electrons and bond type covalent or molecular hydrides are classified into -. So, match each hydrides accordingly we get, a - iv , b - i , c - ii , d - iii. Last updated on Jan 2, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams. Engineering Recruitment Exams. Defence Exams.
Navy AA. UP police computer operator. RPSC Programmer.
.
Not only the metal hydrides are needed as synthetic reagents for preparing the transition metal organometallic compounds but they also are required for important hydride insertion steps in many catalytic processes. The first transition metal hydride compound was reported by W. Heiber in when he synthesized Fe CO 4 H 2. The metal hydride moieties are easily detectable in 1 H NMR as they appear high field of TMS in the region between 0 to 60 ppm, where no other resonances appear. The hydride moieties usually couple with metal centers possessing nuclear spins. A limitation of neutron diffraction method is that large sized crystals are required for the study. Protonation reactions For this reaction to occur the metal center has to be basic and electron rich. From hydride donors Generally for this method, a main group hydride is reacted with metal halide. Using dihydrogen H 2 addition This method involves oxidative addition of H 2 and thus requires metal centers that are capable of undergoing the oxidative addition step. Metal hydrides are reactive species kinetically and thus participate in a variety of transformations like the ones discussed below.
Electron rich hydrides
Hydride , in simple terms, is said to be the anion of hydrogen. It is a chemical compound where the hydrogen atoms exhibit nucleophilic, basic or reducing properties. Compounds of hydrogen with less electronegative elements are known as hydrides. So, when hydrogen reacts with any other element, the product formed is considered to be a hydride. If we closely observe the periodic table, hydride formation is not seen from VA group elements, and this condition is known as the hydride gap. Hydrogen molecule usually reacts with many elements except noble gases to form hydrides.
Pandora ring 52 size
BPSC Assistant. Bihar TET. Punjab Police Constable. Punjab Police SI. EMRS Librarian. BIS Stenographer. Define the following terms: a electron-deficient b electron - precise c electron-rich compound. Territorial Army. AP High court Stenographer. Bombay High Court Peon. DHS Assam Grade 3. Judiciary Exams.
As described in Section 8. In the case of neutral atomic hydrogen, this orbital is occupied by one electron.
GATE Chemistry. Cod liver oil obtained from fish is rich in:. Assam Forest Ranger. MP Civil Judge. KSP Constable. AOC Material Assistant. Kerala SET. Maharashtra Forest Guard. BSF Head Constable. Example: NH 3 , PH 3 , etc. Allahabad High Court Group C.

Thanks for the help in this question, the easier, the better �
Thanks for the valuable information. It very much was useful to me.
Your idea is magnificent