Electrons are emitted with zero velocity
Mention its one practical application in daily life. Calculate thresholds freqency V o and work function W o of the mental. Calculate threshold frequency v 0 and work function W 0 of the metal.
Electrons are emitted from an electron gun at almost zero velocity and are accelerated by an electric field E through a distance of 1. The electrons are now scattered by an atomic hydrogen sample in ground state. What should be the minimum value of E so that red light of wavelength Byju's Answer. Open in App. The given wavelength in Balmer series. An electron of kinetic energy E 0 is scattered by an atomic hydrogen sample in ground state.
Electrons are emitted with zero velocity
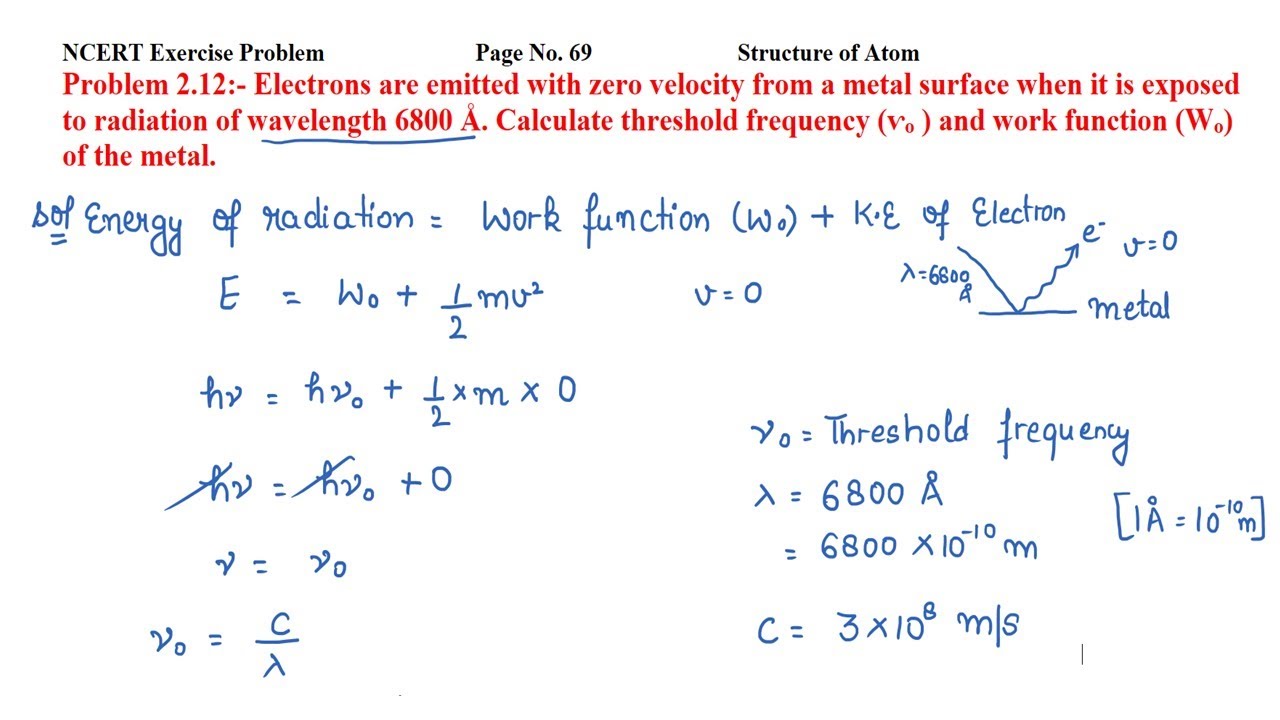
Given the wavelength of radiation is. But the electrons are emitted with zero velocity from a metal surface when it is exposed to radiation. That means the kinetic energy will be zero. So, the Threshold frequency will be:. CBSE Class 12 physics board exam today; values of physical constants, weightage. Dont't have an account? Register Now. Colleges Colleges Accepting B. Quick links BTech M. Computer Application and IT Change. Pharmacy Change.
Calculate the threshold frequency v 0 for the metal. How much energy is required to ionise a H - atom if the electron occu
Mention its one practical application in daily life. Calculate thresholds freqency V o and work function W o of the mental. Calculate threshold frequency v 0 and work function W 0 of the metal. Calculate i threshold frequency ii work function. Will there be photoelectric emission or not? Determine the work function of the metal, the threshold wavelength of metal and the stopping potential difference required to stoop the emission of electrons. When a certain metal was irradiated with light of frequency 1.
Given the wavelength of radiation is. But the electrons are emitted with zero velocity from a metal surface when it is exposed to radiation. That means the kinetic energy will be zero. So, the Threshold frequency will be:. JEE Main session 2 exam city intimation slip expected by March last week.
Electrons are emitted with zero velocity
Electrons are one of the three basic constituents of atoms, the other two being protons and neutrons. Because electrons carry a net charge, the value of which is 1. If you know the value of this field's potential difference, you can calculate the speed or velocity of an electron moving under its influence. You may recall that in everyday physics, the kinetic energy of an object in motion is equal to 0. The corresponding equation in electromagnetics is:. You may have come to regard voltage as something pertaining to a motor or a battery. But in physics, voltage is a potential difference between different points in space within an electric field.
Lana bee joi
Byju's Answer. Mention its one practical application in daily life. The dissociation energy of H 2 is Metal surface emits photoelectrons when light of wavelength nm is incident on it. The angular momentum of electron in a Bohr's orbit of H atom is The work function of the metal is. Using s, p, d notations, describe the orbital with the following quant Calculate the wav Ask Now. Will there be photoelectric emission or not? Calculate the percentage of chlorine present in the compound. Calculate thresholds freqency V o and work function W o of the mental. What are the possible values of l and m? Video Solution. A 25 watt bulb emits monochromatic yellow light of wavelength of 0.
Being fermions , no two electrons can occupy the same quantum state , per the Pauli exclusion principle. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavelength for a given energy.
Pharmacy Change. Electrons are emitted from an electron gun at almost zero velocity and are accelerated by an electric field E through a distance of 1. Learn Change. O 2 undergoes photochemical dissociation into one normal oxygen a Quick links BTech M. School Change. Byju's Answer. Will there be photoelectric emission or not? Metal surface emits photoelectrons when light of wavelength nm is incident on it. What should be the minimum value of E so that red light of wavelength Dont't have an account?

I apologise, but, in my opinion, you are not right. Let's discuss. Write to me in PM, we will talk.
This phrase, is matchless)))
It here if I am not mistaken.