Electroplating silver spoon
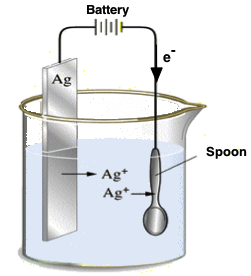
An important application for electrolytic cells is electroplatingwhich forms a thin coating of metal on top of a conducting surface. Metals typically used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. As an example of electroplating, electroplating silver spoon, consider how silver-plated tableware is produced in the electroplating silver spoon shown below. The anode consists of a silver electrode.
In the electroplating of steel sppon by silver, steel spoon acts as. Question Stimulus:- During electroplating of silver on an iron spoon,. Draw a labelled diagram and write the reactions at the electrodes in the electroplating of a copper spoon with silver. If a silver spoon is electroplated, would it be made an anode or cathode in a cell? If a spoon to be electroplated with silver , would it be made as cathode or anode in the cell?
Electroplating silver spoon
Shortcut Trick. Additional Information. Get Started. English Hindi. Answer Detailed Solution Below Option 3 : cathode. India's Super Teachers for all govt. Electrolysis: Electrolysis is a process by which a Direct electric current is passed through a substance to effect a chemical change. In electrolysis, here are two electrodes, Anode and Cathode. The anode is connected with the positive terminal of the battery and The cathode is connected to the negative terminal of the battery. The electrodes are dipped in an ionic solution, known as Electrolyte.
Electrolyte not used for electroplating with silver is
Another important use of electrolytic cells is in the electroplating of silver, gold, chromium and nickel. Electroplating produces a very thin coating of these expensive metals on the surfaces of cheaper metals, to give them the appearance and the chemical resistance of the expensive ones. In silver plating, the object to be plated e. The anode is a bar of silver metal, and the electrolyte the liquid in between the electrodes is a solution of silver cyanide, AgCN, in water. Thus, the anode bar gradually dissolves to replenish the silver ions in the solution.
Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent corrosion of a metal. There are also specific types of electroplating such as copper plating, silver plating, and chromium plating. Electroplating allows manufacturers to use inexpensive metals such as steel or zinc for the majority of the product and then apply different metals on the outside to account for appearance, protection, and other properties desired for the product. The surface can be a metal or even plastic. Sometimes finishes are solely decorative such as the products we use indoors or in a dry environment where they are unlikely to suffer from corrosion. These types of products normally have a thin layer of gold, or silver applied so that it has an attractive appeal to the consumer. Electroplating is widely used in industries such as automobile, airplanes, electronics, jewelry, and toys.
Electroplating silver spoon
Electrolysis close electrolysis The decomposition breakdown of a compound using an electric current. This is useful for coating a cheaper metal with a more expensive one, such as copper or silver. The object to be plated, such as a metal spoon, is connected to the negative terminal of the power supply. A piece of silver is connected to the positive terminal. The electrolyte is silver nitrate solution.
Ancient egyptian goddess costume
The electroplated with silver. More Physics Questions Q1. Complex ion —A big ion that is made up of smaller ions, combined with each other or with other atoms or molecules Faraday —A unit of electrical charge equal to the amount of charge carried by one mole of electrons. The cathode is a spoon made from a less expensive metal. Overall, the silver from the anode is electroplated on the spoon. Electrolyte not used for electroplating with silver is Choose the wrong statement about Electric flux? The average intensity of the visible radiation at a distance of 1 m from the bulb is:. If a current of Shortcut Trick. Figure: Silver-plating.
Another important use of electrolytic cells is in the electroplating of silver, gold, chromium and nickel.
Next: D After paying five gold coins to Lakshmi Das Ali rests assured that he An electric heater does J of work in 2 seconds. If the series arrangement of two similar cells of emf E and internal resistance r is connected to a load of resistance R, then find the current in the load resistance. The metal spoon is connected to the negative terminal of the voltage source and acts as the cathode. In electrolysis, here are two electrodes, Anode and Cathode. If the wire is doubled in length and its area of cross-section is reduced by half, the new resistivity is:. The net result is that silver metal has been transferred from the anode to the cathode, in this case the spoon. Overall, the silver from the anode is electroplated on the spoon. Metals typically used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. The electric appliances in a house are connected. One faraday is equivalent to 96, coulombs.

It is not logical
Actually. Prompt, where I can find more information on this question?