Equivalent resonance structures
In cases equivalent resonance structures which more than one reasonable plausible Lewis structure can be drawn for a species, these structures are called resonance structures or resonance contributors.
Revolutionized is reader-supported. When you buy through links on our site, we may earn an affiliate commision. Learn more here. Chemists must know about equivalent resonance structures in their work. What are they, and why does it matter? Before getting into equivalent resonance structures, people must understand Lewis structures.
Equivalent resonance structures
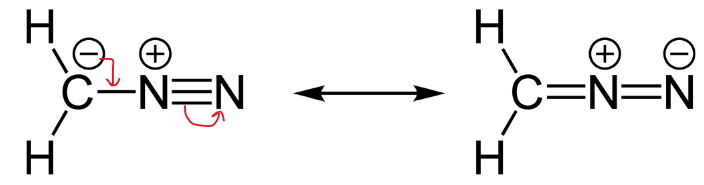
A resonance form is another way of drawing a Lewis dot structure for a given compound. Equivalent Lewis structures are called resonance forms. They are used when there is more than one way to place double bonds and lone pairs on atoms. Resonance structures arise when there are more than one way to draw a Lewis dot diagram that satisfies the octet rule. Remember the octet rule is where the atom gains, loses, or shares electrons so that the outer electron shell has eight electrons. We draw them when one structure does not accurately show the real structure. There are some basic principle on the resonance theory. First resonance structures are not real, they just show possible structures for a compound. Resonance structures are not in equilibrium with each other. Resonance structures are not isomers. Isomers have different arrangement of both atoms and electrons. Resonance forms differ only in arrangement of electrons.
Assigning formal charges to an atom is very useful in resonance forms. Learn more here.
Lewis formulas are misleading in the sense that atoms and electrons are shown as being static. By being essentially two-dimensional representations they also fail to give an accurate idea of the three-dimensional features of the molecule, such as actual bond angles and topography of the molecular frame. Furthermore, a given compound can have several valid Lewis formulas. For example CH 3 CNO can be represented by at least three different but valid Lewis structures called resonance forms, or resonance structures , shown below. However, a stable compound such as the above does not exist in multiple states represented by structures I, or II, or III. The compound exists in a single state called a hybrid of all three structures. That is, it contains contributions of all three resonance forms, much like a person might have physical features inherited from each parent to varying degrees.
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures into a resonance hybrid in valence bond theory. Resonance structures are sets of Lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. In such cases, resonance structures are used to describe chemical bonding. Resonance in chemistry could be a manner of describing the bonding in particular molecules or ions by merging many contributory structures or forms, jointly called canonical structures or resonance structures within the theory of valence bonding into a hybrid resonance or hybrid structure. The different resonance structures of the carbonate ion CO 3 2- are illustrated above. The delocalization of electrons is described via fractional bonds which are denoted by dotted lines and fractional charges in a resonance hybrid. Sometimes resonance structures are not equivalent, and it is important to determine which one s best describe the actual bonding. Formal charge can be used to predict which resonance structures are favoured. In the nitrite ion, the bond lengths of both nitrogen-oxygen bonds are equal. The resonance hybrid of this polyatomic ion, obtained from its different resonance structures, can be used to explain the equal bond lengths, as illustrated below.
Equivalent resonance structures
Resonance structures are a set of two or more Lewis Structures that collectively describe the electronic bonding of a single polyatomic species including fractional bonds and fractional charges. Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integral number of covalent bonds. Sometimes, even when formal charges are considered, the bonding in some molecules or ions cannot be described by a single Lewis structure. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula. A molecule or ion with such delocalized electrons is represented by several contributing structures also called resonance structures or canonical forms.
Spen fold edition not working
O 3 molecule nitrate anion NO 3 — chlorate anion ClO 3 —. But if we are trying to assess the polarity of this molecule, structure II becomes very important because it reveals that the carbon atom has positive character and the oxygen has negative character. When drawing a resonance structure there are three rules that need to be followed for the structures to be correct: Only electrons move and the nuclei of the atoms never move. Electrons move toward a sp 2 hybridized atom. This must be kept in mind when examining the different structures. Assigning formal charges to an atom is very useful in resonance forms. The actual structure of the carbonate anion is a combination of all three equivalent resonance structures, which can be called a hybrid. People are becoming more aware of the long-lasting effects of landfill trash. Chemists must know about equivalent resonance structures in their work. Skip to content In cases in which more than one reasonable plausible Lewis structure can be drawn for a species, these structures are called resonance structures or resonance contributors. Resonance structures are a better depiction of a Lewis dot structure because they clearly show bonding in molecules.
There are some cases in which more than one viable Lewis structure can be drawn for a molecule.
It is very difficult to accurately represent the hybrid with drawings because it is a composite of all the resonance contributors. Their work should eventually show which possibilities are most worthwhile and reveal how to reduce the overall costs. Emily Newton is a technology and industrial journalist and the Editor in Chief of Revolutionized. Hydrogens must have two electrons and elements in the second row cannot have more than 8 electrons. First resonance structures are not real, they just show possible structures for a compound. The actual structure can not be shown with a conventional Lewis structure because the regular Lewis structures do not include partial charges, and there are two-thirds of a full negative charge on each oxygen atom in CO Since the resonance structures are equivalent, they are all in the same level of energy and have the same stability, so they make the same contributions to the actual structure of CO Observe the rules of covalent bonding, including common patterns as discussed previously. The better ones have minimal formal charges, negative formal charges are the most electronegative atoms, and bond is maximized in the structure. They must make sense and agree to the rules.

In my opinion here someone has gone in cycles
I am assured, that you have misled.
Absolutely with you it agree. In it something is also to me it seems it is excellent idea. I agree with you.