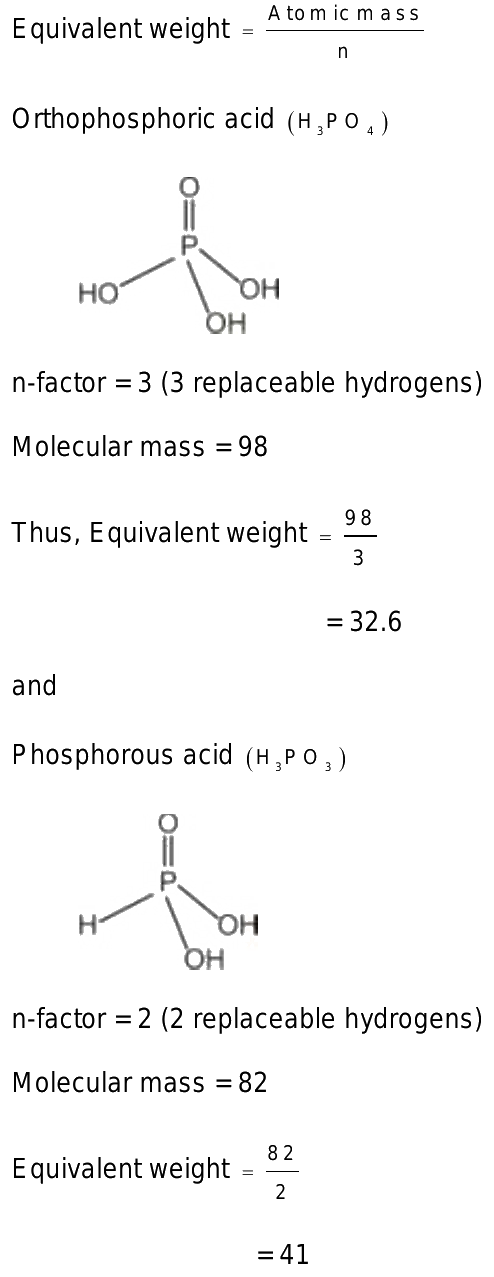
Equivalent weight of phosphoric acid
Aby znaleźć odpowiednią aplikację, należy użyć filtrów branż i próbek lub użyć wyszukiwania tekstowego.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. In recent years, there has been a continuous increase in the incidence of urolithiasis, especially in highly developed countries. Therefore, the question arises which factors specific to these countries may be responsible for the increase in the incidence of this disease.
Equivalent weight of phosphoric acid
Incorporating a sensor with completely PFA perfluoroalkoxy wetted parts, size is optimized to the absolute limit. Employing a sensor with a sanitary structure, using only PFA as the wetted material. Being a chemically resistant sensor, it is capable of measuring various chemicals used in semiconductor processes. Concentration conversion is possible by inputting the relationship between chemical concentration and conductivity along with temperature characteristics. It is particularly suitable for the dilution management of low-concentration chemical solutions. Masz pytania lub prośby? Skorzystaj z tego formularza, aby skontaktować się z naszymi specjalistami. Segment: Semiconductor. Dział: Wet Process Control. Contamination-free Employing a sensor with a sanitary structure, using only PFA as the wetted material. Concentration conversion function Concentration conversion is possible by inputting the relationship between chemical concentration and conductivity along with temperature characteristics. Wniosek o udzielenie informacji Masz pytania lub prośby?
Sorry, a shareable link is not currently available for this article.
Use of this information is subject to copyright laws and may require the permission of the owner of the information, as described in the ECHA Legal Notice. EC number: CAS number: For the inhalation route there is no animal study available. Therefore, oral rat data is used to calculate a corresponding air concentration for humans and a route-to-route extrapolation for systemic effects is necessary to derive the correct starting point. In the case of oral-to-inhalation the inclusion of a default factor of 2 is recommended according to chapter R. According to Figure R.
Phosphoric acid orthophosphoric acid, monophosphoric acid or phosphoric V acid is a colorless, odorless phosphorus -containing solid , and inorganic compound with the chemical formula H 3 P O 4. It is a major industrial chemical, being a component of many fertilizers. The compound is an acid. Phosphoric acid forms esters , called organophosphates. The name "orthophosphoric acid" can be used to distinguish this specific acid from other " phosphoric acids ", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature. Phosphoric acid is produced industrially by one of two routes, wet processes and dry. In the wet process, a phosphate-containing mineral such as calcium hydroxyapatite and fluorapatite are treated with sulfuric acid. Calcium sulfate gypsum, CaSO 4 is a by-product, which is removed as phosphogypsum. The hydrogen fluoride HF gas is streamed into a wet water scrubber producing hydrofluoric acid.
Equivalent weight of phosphoric acid
Dear student, you haven't completed the reaction part, but don't worry I know about the reaction of H3PO4 with NaOH, this reaction is generally asked by students. We know that for the Acids, the equivalent weight is equal to the ratio of the molar mass and number of the hydrogen ions of the acid accepted by the base. We endeavor to keep you informed and help you choose the right Career path. When you look back in life , this app would have played a huge role in laying the foundation of your career decisions. Found everything I wanted and it solved all of my queries for which I was searching a lot A must visit No need to find colleges in other sites, this is the best site in India to know about any colleges in India.
Crystal cruises casino
Plate was incubated and after 24 h at 37 °C bacterial growth was observed. Oferujemy całą gamę praktycznych akcesoriów — od supernowoczesnych roz The course of struvite crystals growth in artificial urine with different phosphorus concentration and increasing pH. The numbers refer to the numbering of the photos in a and correspond to the concentrations of phosphorous. Phosphoric Acid. Hydrochloric Acid. Citric Acid. Albert Bartens KG. Community methods for the analysis of wines - comparison between hydrostatic balance and electronic densimetry, proving them as equivalent. The results of spectrophotometric measurements are presented in Fig. The obtained results indicate that phosphoric acid present in artificial urine causes the nucleation of struvite to shift towards a lower pH, which means that struvite nucleates earlier in artificial urine compared to the control test.
Phosphoric acid is a colorless, odorless, inorganic compound. It is represented by the chemical formula H 3 PO 4. It is extensively used in distinct fields.
Article ID , 7. In the case of phosphorus concentrations 2, 3 and 4, the increase in absorbance occurs at even lower pH values Fig. The question is: what factors are associated with the progressive increase in the incidence of infectious urolithiasis in highly developed countries? Uncertainty of measurement in clinical microbiology. The recommended consumption of phosphorus in healthy people over 18 years of age, regardless of sex, including breastfeeding and pregnant women, is mg 37 per day, regardless of body weight. Hydrochloric Acid. First, it can be seen that phosphoric acid lowers the initial pH of the urine. The tested concentrations 1, 2, 3 and 4 Table 2 result from the recommended, average, permissible and maximum phosphorus consumption, respectively. The elevated phosphorus concentration tested was equal to Chemistry and mechanism of urease inhibition. Microscopic examination of thyroid, lung, heart, liver, spleen, kidneys, adrenals, stomach, pancreas, bone marrow, large and small intestine uterus, ovaries and testes on a representative number of animals no further information was performed. Google Scholar Bazin, D.

What words... super, a brilliant phrase