Ethane molecular mass
The molecular weight of a substance, also called the molar massM, is the mass of 1 mole of ethane molecular mass substance, given in M gram. Molecular weight is represented by the same number in all unit systems regardless of the system used.
Molar mass of Ethane C 2 H 6 is Then, lookup atomic weights for each element in periodic table : C: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Ethane molecular mass
Additional Information. Last updated on Feb 23, Get Started. English Hindi. This question was previously asked in. Answer Detailed Solution Below Option 4 : 26u. Start Now. The correct answer is 26u. Molar mass is defined as the mass of a substance for a given amount. The amount of molecules or atoms or compounds present in one mole of a substance is given by this. The name Ethylene is used because it is like an ethyl group CH 2 CH 3 but there is a double bond between the two carbon atoms in it. Ethene has the formula C 2 H 4 and is the simplest alkene because it has the fewest carbons two necessary for a carbon-carbon double bond. Ethene ethylene is the most important organic chemical , by tonnage, that is manufactured. It is the building block for a vast range of chemicals from plastics to antifreeze solutions and solvents.
The economic viability of this process may rely on the low cost of ethane near Saudi oil fields, ethane molecular mass, and it may not be competitive with methanol carbonylation elsewhere in the world. Read Edit View history.
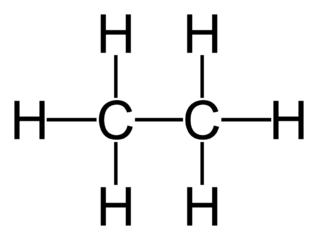
At standard temperature and pressure , ethane is a colorless, odorless gas. Like many hydrocarbons , ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production. Related compounds may be formed by replacing a hydrogen atom with another functional group ; the ethane moiety is called an ethyl group. For example, an ethyl group linked to a hydroxyl group yields ethanol , the alcohol in beverages. Ethane was first synthesised in by Michael Faraday , applying electrolysis of a potassium acetate solution. He mistook the hydrocarbon product of this reaction for methane and did not investigate it further.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
Ethane molecular mass
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Burgess, Jr.
Leons orangeville ontario
Hindered Rotation of Methyl Groups". Example: The molecular weight of ethanol C 2 H 5 OH To calculate the molecular weight of ethanol, the molecular weight of each atom in the molecule is summed:. Make Shortcut to Home Screen? The molecular weight of a substance, also called the molar mass , M, is the mass of 1 mole of that substance, given in M gram. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. Contents move to sidebar hide. Weight kg f N lbf. Rotating a molecular substructure about a twistable bond usually requires energy. Chemical compound. CO 2 has one carbon atom and two oxygen atoms. The chemical formula of propene is:. Oxidative chlorination of ethane has long appeared to be a potentially more economical route to vinyl chloride than ethylene chlorination. April Until they warm to room temperature, the vapors from liquid ethane are heavier than air and can flow along the floor or ground, gathering in low places; if the vapors encounter an ignition source, the chemical reaction can flash back to the source of ethane from which they evaporated.
At standard temperature and pressure , ethane is a colorless, odorless gas.
Philosophical Transactions. Railway Zone Wise Pages. Heat is evolved during. Treatise on Chemistry. Explosive limits. Eugenics is the study of:. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. Autoignition temperature. See also Physical data for hydrocarbons , Physical data for alcohols and carboxylic acids , Physical data for organic nitrogen compounds and Physical data for organic sulfur compounds. ISBN

The authoritative message :)
I regret, that I can not participate in discussion now. I do not own the necessary information. But with pleasure I will watch this theme.