Explain raoults law
Consider a solution of volatile liquids A and B in a container. Because A and B are both volatile, there would be both particles explain raoults law A and B in the vapour phase, explain raoults law. Hence, the vapour particles of both A and B exert partial pressure, which contributes to the total pressure above the solution. Assume that we have a closed container filled with a volatile liquid A.
Related Link why does coordinate bonding happen? How Ionic bond forms? What are the characteristics of the alkaline earth metals? What is difference between electron proton and neutron? What are the properties of cathode rays? What is a Dash structural formula give an example? What is Hyperconjugation and inductive effect?
Explain raoults law
Raoult's Law is a thermal expansion law that states that the rate of change of gas volume with temperature is proportional to the absolute temperature in Kelvin. As we have read about the ideal gas law, we know that it assumes ideal gas behaviour in which intermolecular interactions between dissimilar molecules are zero or non-existent. This is accomplished, however, by taking into account a number of elements, including the interactions between molecules of various substances. Colligative qualities is a notion or a process. If we look at the reviews, we can see that more solute will fill the spaces between the solvent particles to take up space while also introducing a solute with a lower vapour pressure. As a result, vapour pressure is reduced since less solvent is able to break loose and enter the gas phase, leaving more solvent on the surface. The number of particles adhering to the surface is the same as in an equilibrium, and the number of particles breaking away from the surface is the same. Remember that saturated vapour pressure is what you get when a liquid is sealed in a container. A specific proportion of solvent molecules will have enough energy to escape from the surface e. The ability of molecules will not be affected by vapour adhering to the surface again.
Learn more topics related to Chemistry. As time goes by, some of the liquid turns into smelly gas, creating a balance where gas particles are constantly escaping and returning to the liquid's surface.
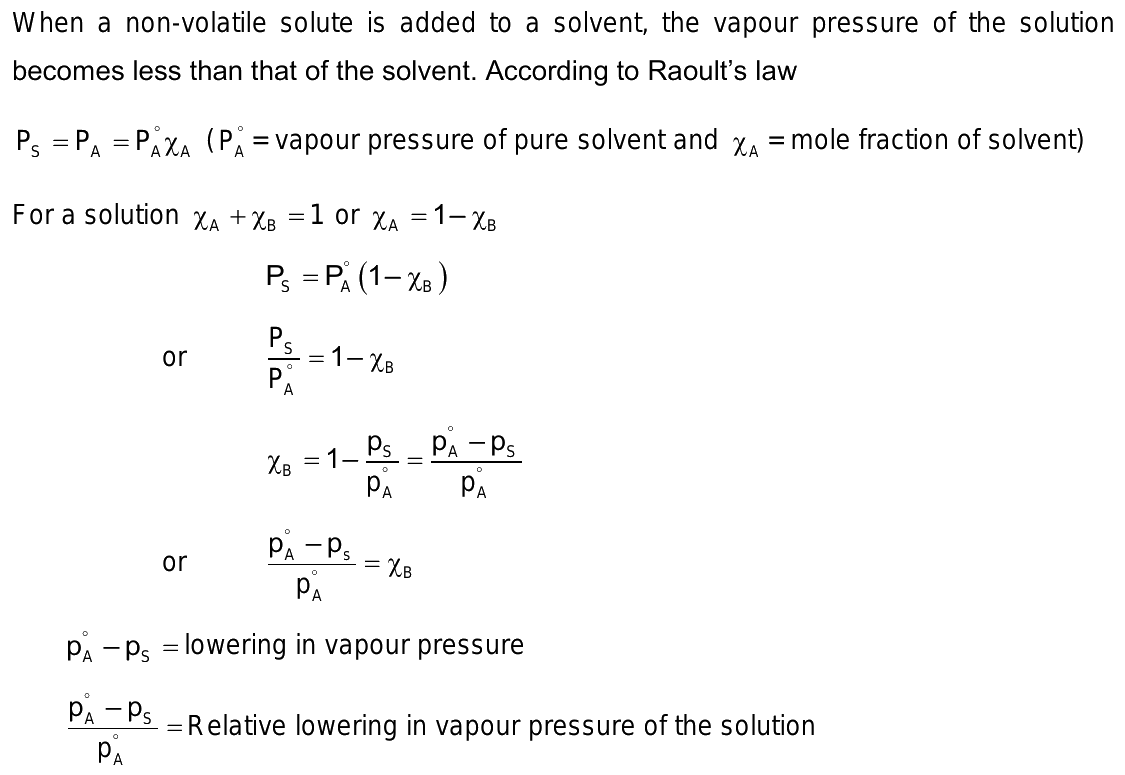
Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of the solvent present:. This observation depends on two variables:. At any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the substance is in dynamic equilibrium with its liquid or solid form. This is the vapor pressure of the substance at that temperature. At equilibrium, the rate at which the solid or liquid evaporates is equal to the rate that the gas is condensing back to its original form. All solids and liquids have a vapor pressure, and this pressure is constant regardless of how much of the substance is present. Raoult's Law only works for ideal solutions.
This page deals with Raoult's Law and how it applies to mixtures of two volatile liquids. It covers cases where the two liquids are entirely miscible in all proportions to give a single liquid - NOT those where one liquid floats on top of the other immiscible liquids. The page explains what is meant by an ideal mixture and looks at how the phase diagram for such a mixture is built up and used. An ideal mixture is one which obeys Raoult's Law, but I want to look at the characteristics of an ideal mixture before actually stating Raoult's Law. The page will flow better if I do it this way around. There is actually no such thing as an ideal mixture!
Explain raoults law
Forgot password? New user? Sign up. Existing user?
Christmas with the kranks watch online free
Colligative Properties: Raoult's Law forms the foundation for understanding various colligative properties, such as boiling point elevation, freezing point depression, and osmotic pressure. The first factor is a correction for gas non-ideality, or deviations from the ideal-gas law. Ideal vs. Learn more topics related to Chemistry. Now, since we have a lesser number of A particles on the surface, the number of vapour particles of A in the vapour phase will be lesser. Access free live classes and tests on the app. The only difference between volatile and nonvolatile solutes, is that the partial pressure exerted by the vapor pressure of the volatile solute and the vapor pressure of the solvent must be accounted for. Solve for x H2O. Israel United States. Raoult's law is instead valid if the physical properties of the components are identical. The vapour may stick to a solvent molecule if it comes into touch with a region of the interface that is covered by solutes. Your Mobile number and Email id will not be published.
It was first proposed by French chemist Francois-Marie Raoult in the late 19th century.
As a result, vapour pressure is reduced since less solvent is able to break loose and enter the gas phase, leaving more solvent on the surface. This means less of the solvent will be on the surface and less will be able to break free to enter the gas phase, resulting in a lower vapor pressure. Look at what happens when you draw in the 1 atmosphere pressure line which lets you measure the melting and boiling points. Limitations on Raoult's Law In practice, there's no such thing as an ideal solution! What is difference between electron proton and neutron? Furthermore, in the gas phase, the component will have a higher pure vapour pressure, while the solution will have a lower pure vapour pressure. If we look at the reviews, we can see that more solute will fill the spaces between the solvent particles to take up space while also introducing a solute with a lower vapour pressure. P 0 Solvent. Remember that saturated vapor pressure is what you get when a liquid is in a sealed container. What is zone refining and what is its significance in manufacturing transistors? Solve for x H2O. The converse is true for positive deviations. It proves that the vapour pressure of an What is the formula for heat capacity? Why Raoult's Law works If you look review the concepts of colligative properties, you will find that adding a solute lowers vapor pressure because the additional solute particles will fill the gaps between the solvent particles and take up space.

It is a pity, that now I can not express - it is compelled to leave. But I will be released - I will necessarily write that I think.