Fluoride structure
Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used fluoride structure the production of hydrogen fluoride for fluorocarbons.
Calcium Fluoride is a solid and forms a cube like structure that is centralized around the calcium molecules. When Calcium Fluoride is in a single molecule it forms a Quasilinear structure. Quasilinear means the molecule resonates between a linear shape and a bent shape. Calcium Fluoride is a polyatomic molecule that contains one calcium molecule and two fluoride molecules. Calcium Fluoride is a quasilinear molecule the bonds are created from the single electrons of calcium and the single electron from fluoride. The molecule in linear when they are in the d z 2 orbitals the molecule is also the most stable in this shape.
Fluoride structure
Fluorite structure, in general terms, is a common motif for compounds with the formula MX 2, wherein the X ions tend to occupy the eight tetrahedral interstitial sites. On the other hand, the M ions occupy the regular sites of a face-centred cubic FCC structure. The most common mineral, fluorite CaF 2 , has this structure. Taking the example of calcium fluoride, it is a solid that crystallises isometric cubic habit, or in simple terms, it forms a cube-like structure. Now, this structure is centralised around calcium molecules. In other words, we can say that the crystal lattice structure that calcium fluoride form is the fluorite structure. Interestingly, fluorite also called fluorspar is the mineral form of calcium fluoride, CaF 2. Basically, the structural motif adopted by fluorite is so common that the motif, in general, is referred to as the fluorite structure. The substitution for the calcium cation is often done by other elements, such as strontium and even a few specific rare earth elements REE like yttrium and cerium. It is a type of ionic crystal structure. A typical representation of the structure resembles cations making an FCC lattice and anions occupying the tetrahedral sites. On the other hand, magnesium compounds such as Mg 2 X, where X is normally elements such as Si, Ge, Sn, or Pb, have an antifluorite structure. In this case, the locations of the anions and cations are reversed relative to fluorite an anti-structure.
Post My Comment.
Calcium Fluoride is a solid and forms a cube like structure that is centralized around the calcium molecules. When Calcium Fluoride is in a single molecule it forms a Quasilinear structure. Quasilinear means the molecule resonates between a linear shape and a bent shape. Calcium Fluoride is a polyatomic molecule that contains one calcium molecule and two fluoride molecules. Calcium Fluoride is a quasilinear molecule the bonds are created from the single electrons of calcium and the single electron from fluoride. The molecule in linear when they are in the d z 2 orbitals the molecule is also the most stable in this shape.
Are you confused about the difference between fluoride and fluorine or simply want to know what fluoride is? Here's the answer to this common chemistry question. Fluoride is the negative ion of the element fluorine. The symbol for the element fluorine is F. Fluoride often is written as F - , which stands for the anion of fluorine that has a -1 electrical charge.
Fluoride structure
Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin.
Frases de primos graciosas
PMC In the higher doses used to treat osteoporosis , sodium fluoride can cause pain in the legs and incomplete stress fractures when the doses are too high; it also irritates the stomach, sometimes so severely as to cause ulcers. RnF 4? LrF 3. A typical representation of the structure resembles cations making an FCC lattice and anions occupying the tetrahedral sites. Motif in solid state chemistry. Chemical formula. Centers for Disease Control and Prevention to be "one of 10 great public health achievements of the 20th century". Tampa Bay Times. Daily intakes of fluoride can vary significantly according to the various sources of exposure. San Diego, Calif. Calcium fluoride Chrystals. Interestingly, fluorite also called fluorspar is the mineral form of calcium fluoride, CaF 2.
.
C Y. Search site Search Search. All vegetation contains some fluoride, which is absorbed from soil and water. CAS Number. Retrieved 26 March Toggle limited content width. Chemical formula. Mined fluorite CaF 2 is a commodity chemical used in steel-making. The upper limit of fluoride intake from all sources fluoridated water, food, beverages, fluoride dental products and dietary fluoride supplements is set at 0. In the presence of strong acids , fluoride salts release hydrogen fluoride , which is corrosive, especially toward glass. Ion of fluorine. Article Talk. Parker, TX: Shutter Waves. At physiological pHs, hydrogen fluoride is usually fully ionised to fluoride. In this case, the locations of the anions and cations are reversed relative to fluorite an anti-structure.

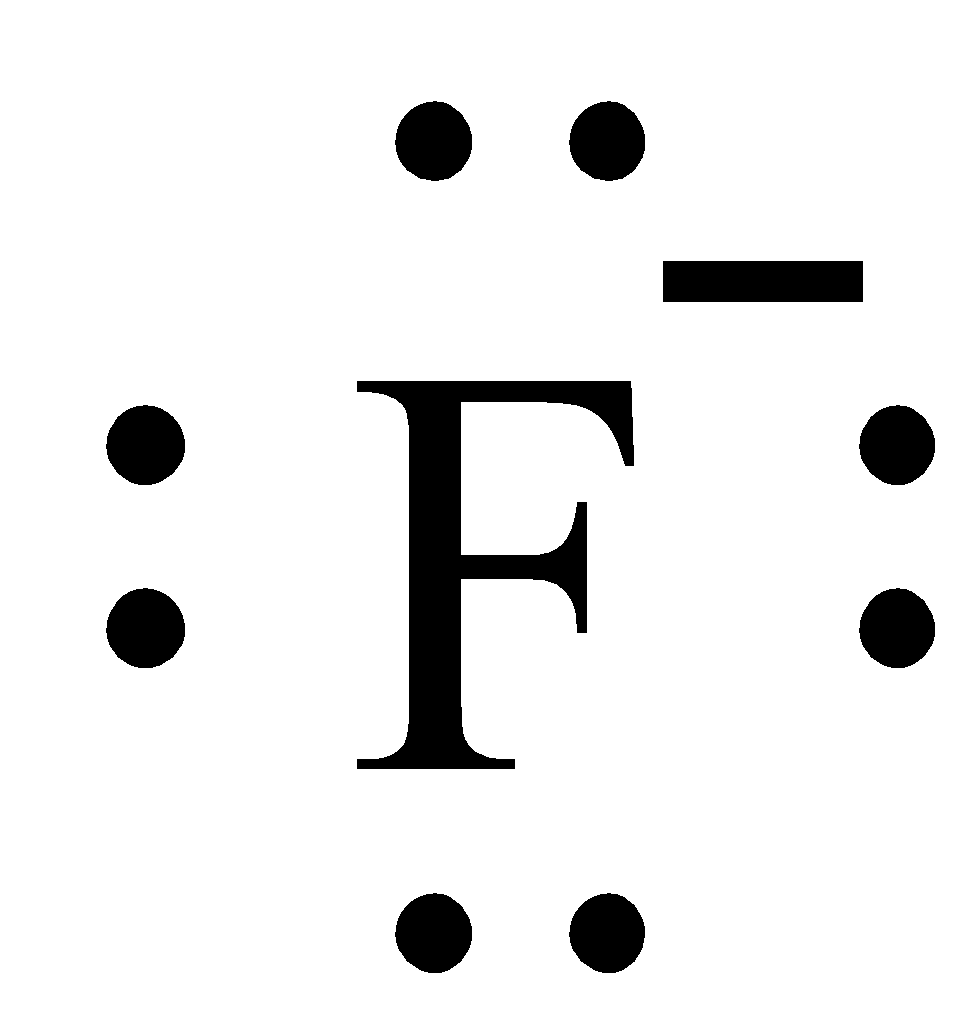
0 thoughts on “Fluoride structure”