H30+ lewis structure
Hydronium ion contains hydrogen and oxygen atoms. Each hydrogen atom has linked with oxygen atom. Only one lone pair exist on oxygen atom.
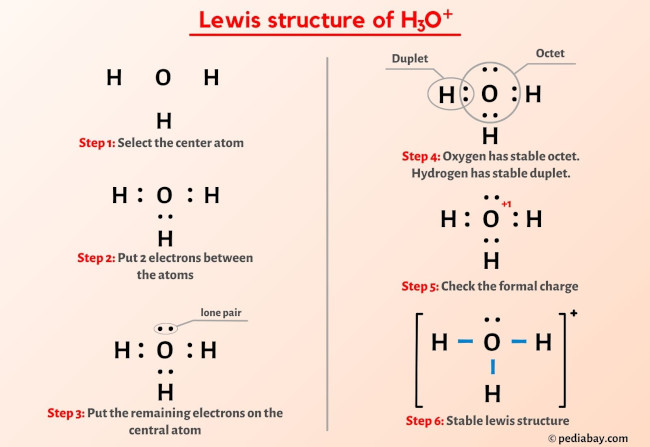
In the periodic table , hydrogen lies in group 1, and oxygen lies in group Hence, hydrogen has one valence electron and oxygen has six valence electrons. Learn how to find: Hydrogen valence electrons and Oxygen valence electrons. We have a total of 8 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here hydrogen can not be the central atom.
H30+ lewis structure
There are 3 single bonds between the Oxygen atom O and each Hydrogen atom H. There is 1 lone pair on the Oxygen atom O. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. You can see the electronegativity values of hydrogen atom H and oxygen atom O in the above periodic table. If we compare the electronegativity values of hydrogen H and oxygen O then the hydrogen atom is less electronegative. But as per the rule we have to keep hydrogen outside. Now in the H3O molecule, you have to put the electron pairs between the oxygen atom O and hydrogen atoms H. This indicates that the oxygen O and hydrogen H are chemically bonded with each other in a H3O molecule.
He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. And when we divide this h30+ lewis structure by two, we get the value of total electron pairs.
.
If we see the nomenclature of hydronium ion, we get to know that according to the IUPAC nomenclature, hydronium ion can be referred to as oxonium. Oxonium is a generalized name for all trivalent oxygen cations, so the use of the name hydronium is necessary to identify hydronium ions particularly. This ion is used in determining the pH of water. The hydronium ion is used in various reactions and the production of different compounds. Both organic and inorganic chemistry includes hydronium ion to a large extent. But before reading the use of this ion in different reactions, we must have knowledge about the basics of this ion, like, lewis structure, geometry, etc. Knowing these basics will deepen our knowledge about this ion more. We should always try to know the background of any compound before studying any reaction regarding it.
H30+ lewis structure
The Oxygen atom O is at the center and it is surrounded by 3 Hydrogen atoms H. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table. Oxygen is a group 16 element on the periodic table.
10 stone converted to pounds
Your email address will not be published. These outer hydrogen atoms are forming a duplet and hence they are stable. We have a total of 8 valence electrons. There are 3 single bonds between the Oxygen atom O and each Hydrogen atom H. Hydrogen is group 1 element on the periodic table. Now, we know how many electrons are there in valence shells of hydrogen and iodine atoms. Oxygen is a group VIA element in the periodic table and contains six electrons in its last shell. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. You can see the number of bonding electrons and nonbonding electrons for each atom of H3O molecule in the image given below. In the periodic table , hydrogen lies in group 1, and oxygen lies in group That means it has 8 electrons. Hence, the octet rule and duet rule are satisfied.
Hydronium ion contains hydrogen and oxygen atoms.
And three O — H bonds are already marked. You can see the number of bonding electrons and nonbonding electrons for each atom of H3O molecule in the image given below. Hydrogen is group 1 element on the periodic table. You have to put these 2 electrons on the central oxygen atom in the above sketch of H3O molecule. Now, we know how many electrons are there in valence shells of hydrogen and iodine atoms. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell. Oxygen is group 16 element on the periodic table. Always start to mark the lone pairs from outside atoms. Here, the outside atoms are hydrogens. Now in the H3O molecule, you have to put the electron pairs between the oxygen atom O and hydrogen atoms H. Hence, hydrogen has one valence electron and oxygen has six valence electrons. Also remember that hydrogen is a period 1 element , so it can not keep more than 2 electrons in its last shell.

Excuse, that I interfere, but I suggest to go another by.