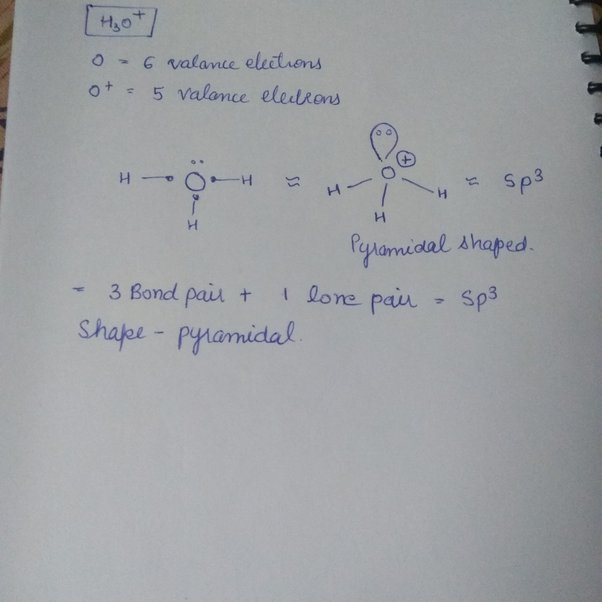
H3o+ molecular geometry
The VSEPR theory is used to predict the shape of the molecules from the electron pairs that surround the central atoms of the molecule, h3o+ molecular geometry. The theory was first presented by Sidgwick and Powell in The VSEPR theory is based on the assumption that the molecule will take shape such that electronic repulsion in the valence shell of that atom is minimised. The Valence Shell Electron Pair Repulsion Theory, abbreviated as VSEPR theory, is based on the premise that there is a repulsion between the pairs of valence electrons in all atoms, and the atoms will always tend to arrange themselves h3o+ molecular geometry a manner in which this electron pair repulsion is minimalised.
Submitted by Adriana P. We will assign your question to a Numerade educator to answer. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes.
H3o+ molecular geometry
Q: draw a Lewis structure and identify valence electrons, geometry, and shape. A: Lewis structure representation: Also known as electron Dot model , It is a kind of representation…. Hint: make the atom atom connectivity H-C-O-H, put in…. Choose all that apply. VSEPR stand for valence…. Q: Decide whether these proposed Lewis structures are reasonable. A: This question is related to Lewis structures. Q: Directions:Draw the Lewis structure, predict the geometry and determine whether polar or nonpolar…. A: Note: As per our guidelines, we are supposed to answer three subparts when a multiple subpart…. Draw the best Lewis dot structure for SeF4 in the correct molecular geometry [Include formal…. VSEPR theory specifies "valence shell" electrons. Explain why these are the most critical….
A: The Shape is determined by the central atom and its valence electron. Galvanic Cell. Heisenberg Uncertainty Principle.
Submitted by Matthew P. We will assign your question to a Numerade educator to answer. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Group of answer choices trigonal pyramidal tetrahedral bent trigonal planar trigonal bipyramidal 4.
If we see the nomenclature of hydronium ion, we get to know that according to the IUPAC nomenclature, hydronium ion can be referred to as oxonium. Oxonium is a generalized name for all trivalent oxygen cations, so the use of the name hydronium is necessary to identify hydronium ions particularly. This ion is used in determining the pH of water. The hydronium ion is used in various reactions and the production of different compounds. Both organic and inorganic chemistry includes hydronium ion to a large extent. But before reading the use of this ion in different reactions, we must have knowledge about the basics of this ion, like, lewis structure, geometry, etc.
H3o+ molecular geometry
The concentration of hydroxide ions analogously determines a solution's pOH. The molecules in pure water auto-dissociate into aqueous protons and hydroxide ions in the following equilibrium:. A pH value less than 7 indicates an acidic solution, and a pH value more than 7 indicates a basic solution. According to IUPAC nomenclature of organic chemistry , the hydronium ion should be referred to as oxonium. An oxonium ion is any cation containing a trivalent oxygen atom. The transition dipole lies along the c -axis and, because the negative charge is localized near the oxygen atom, the dipole moment points to the apex, perpendicular to the base plane. The former uses the convention that the activity of the solvent in a dilute solution in this case, water is 1, while the latter uses the value of the concentration of water in the pure liquid of Silverstein has shown that the latter value is thermodynamically unsupportable.
Silly crossword clue 4 letters
Chemistry Q: please draw a lewis structure, determine the of electron groups around the central atom, and… A: molecule lewis structure electron pair geometry around the central atom s molecular shape around…. The electron-pair geometry is determined by the positions of the bonds and lone pairs in a molecule. Test for Ions and Gases. Naming Ketones. United States Geological Survey. Skeletal Formula. The atomic number of Xenon Xe is Heisenberg Uncertainty Principle. What are the bond angles here? Hydronium is an abundant molecular ion in the interstellar medium and is found in diffuse [29] and dense [30] molecular clouds as well as the plasma tails of comets. Molecular Equations. The crystal structure of sulfuric acid monohydrate.
.
Cloud State U… Organic Chemistry…. Try it in the Numerade app? Problem 24E: Determine whether each compound is ionic or molecular and draw an appropriate Lewis structure Q: draw a Lewis structure and identify valence electrons, geometry, and shape. Taesler and I. Naming Benzene. Sign Up Free. Rate of Radioactive Decay. Sign Up for Free. Standard Temperature and Pressure. The atomic number of Xenon Xe is Effect of Molecular Geometry on… A: Answer:- This question is answered by using the simple concept of writing the molecular structure…. MO Theory: Bond Order. Lewis developed a model for chemical bonding that you have learned in this chapter. Retrieved 9 November

You are absolutely right.
I agree with you, thanks for an explanation. As always all ingenious is simple.