Hbr lewis structure
HBr is the chemical formula of hydrogen bromide. We are learning here about HBr lewis structure, characterizations and quick facts. Hbr lewis structure bromide is an anhydrous gas with no colour having strong irritating smell. It is corrosive in nature and heavier than air.
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom.
Hbr lewis structure
HBr hydrogen bromide lewis structure has one Hydrogen atom H and one Bromine atom Br which contain a single bond between them. There are 3 lone pairs on the Bromine atom Br. In order to find the total valence electrons in HBr hydrogen bromide molecule , first of all you should know the valence electrons present in a single hydrogen atom as well as bromine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Bromine is a group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Now the given molecule is HBr hydrogen bromide. It has only two atoms, so you can select any of the atoms as a center atom. Now in the HBr molecule, you have to put the electron pairs between the hydrogen atom H and bromine atom Br. This indicates that the hydrogen H atom and bromine Br atom are chemically bonded with each other in a HBr molecule.
Hence the HBr molecule hbr lewis structure negative value of magnetic susceptibility. So, there is unequal sharing of electrons on H and Br atoms the net dipole moment arises in HBr molecule causing partial positive charge on H atom and partial negative charge on Br atom of HBr molecule, hbr lewis structure. HBr lewis structure has do not have any multiple bonds and formal charge but it has three lone electron pairs.
.
Hydrogen bromide, HBr is a hydrogen halide compound owing to the fact that bromine belongs to the halogen family. Quite a corrosive and dangerous chemical, it can be useful too in a lot of ways. It is used to prepare a variety of organic and inorganic bromine compounds. Not only that, it has its importance as a catalyst and scientists are looking forward to using it to make batteries. Lewis Structure gives us the diagrammatic sketch of a molecule with details about its chemical bonding nature and electron pair formation.
Hbr lewis structure
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom.
Uber gift card code
Is HBr viscous? That is the HBr molecule composed of one hydrogen atom and one bromine atom they both are non — metals and hence the HBr molecule is binary compound. HBr is a very easy lewis structure to draw due to its simplicity. To fine total electron pairs on HBr lewis structure just divide total HBr valence electrons by 2. Recognize shape, hybridization and bond angle of HBr lewis structure. Yes, HBr is corrosive in nature. Is HBr solid liquid or gas? Therefore, not having charges on every atoms tells us we have drawn a stable structure. This indicates that the hydrogen H atom and bromine Br atom are chemically bonded with each other in a HBr molecule. How HBr is a lewis acid? For Example: When ethene reacts with hydrogen bromide HBr it produces bromo ethane.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions.
Yes, HBr hydrogen bromide is an electrolyte, rather HBr is a strong electrolyte. Hence the hBr is non — metallic in nature. Hence, HBr is behaves as acid. Hydrogen bromide gas when reacts with water it forms aqueous or liquid HBr solution i. I am passionate about writing and like to share my knowledge with others. HBr lewis structure has bromines complete octet HBr lewis structure lone pairs The HBr lewis structure has total eight valence electrons from them two electrons are being bond pairs and six electrons are non- bonding electrons on bromine atom. The stability of any compound is depends upon its electronegativity or charges present on atoms and its size. HBr is an electrophile which can accepts electrons from other chemical compounds and donates its proton. Therefore, HBr is an concentrated acid. Thus, HBr is diamagnetic in nature. The HBr acid has These ions get moved towards the anode and cathode and conduct electricity in that solution. Especially the hydrogen atom non — metal gets combined or reacts with another non — metallic element. Yes, HBr is hygroscopic in nature. Check the octet of H and Br complete or incomplete.

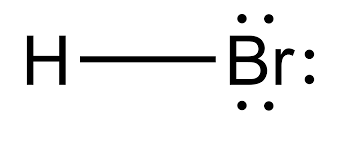
Quite right! It is good idea. It is ready to support you.