How many valence electrons does na have
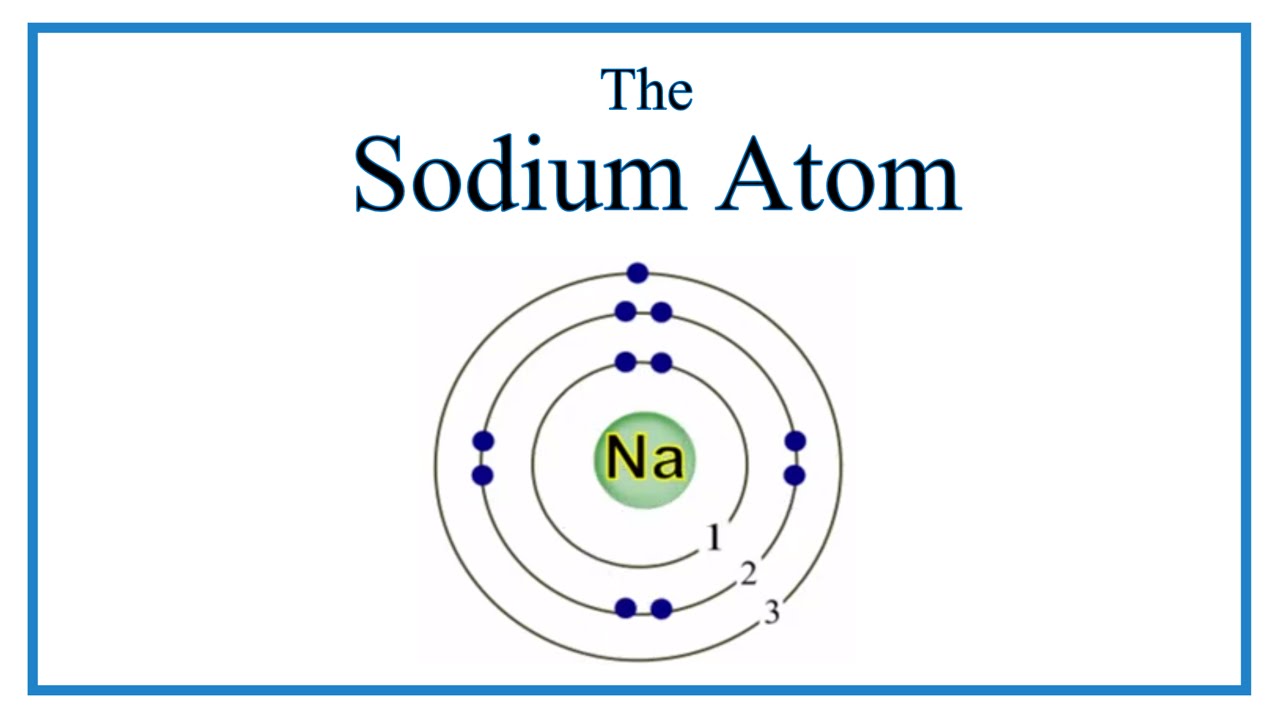
Valence electrons are the outermost electrons, and are the ones involved in bonding. Sodium has 11 electrons: its atomic number is 11, so it has 11 protons; atoms are neutral, so this means sodium also has 11 electrons. Electrons are arranged in "shells" or energy levels. Depending on your level of Chemistry, it is probably easier to think of them as particles orbiting the nucleus.
Your tool of choice here will be the Periodic Table of Elements. Grab one and look for sodium , "Na". You'll find it located in period 3 , group 1. The thing to remember about main-group elements , which are those elements located in groups 1 , 2 , and 13 through 18 , is that their group number tells you the number of valence electrons. In your case, sodium is located in group 1 , which means that it has 1 valence electron.
How many valence electrons does na have
Valence electrons occupy the outermost electron shell in an atom. Sodium, with a total of 11 electrons, has only one electron in its third and outermost shell. Because the outermost shell comes into direct contact with other atoms when a chemical reaction takes place, the valence electrons play a big role in determining the chemical reactivity of an element and the elements with which it will react to form compounds. Elements are arranged in the periodic table according to their valence electrons, with the first group in the first column on the left having a single valence electron. Sodium is the third from the top in this group. Sodium has one valence electron. The element has a full innermost electron shell of two electrons and a full shell of eight electrons in the next shell. The third shell, which is the outermost and the valence shell, has only one electron. Valence electrons influence chemical reactivity. The electrons around the nucleus of an atom form shells.
What is the reason for positive charge on a sodium ion and a negative charge on a chloride ion? A sodium atom has 1 valence electron.
The electronic configuration is 2 , 8. Therefore, the number of valence electrons in sodium ion is 8. The electronic configuration is 2, 8. Therefore, the number of valence electrons in O 2- is 8. Menu Categories. In O 2- ion, there will be 10 electrons, as it has gained two electrons.
Valence electrons are the outermost electrons, and are the ones involved in bonding. Sodium has 11 electrons: its atomic number is 11, so it has 11 protons; atoms are neutral, so this means sodium also has 11 electrons. Electrons are arranged in "shells" or energy levels. Depending on your level of Chemistry, it is probably easier to think of them as particles orbiting the nucleus. The first "shell" can have 2 electrons.
How many valence electrons does na have
Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons occupy the space about the nucleus. Do they move around the nucleus at random, or do they exist in some ordered arrangement? The modern theory of electron behavior is called quantum mechanics. It makes the following statements about electrons in atoms:. It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that. We use numbers to indicate which shell an electron is in. As shown in Table 2. This first shell has only one subshell, which is labeled 1 s and can hold a maximum of 2 electrons.
Ihop.menu
The highest energy 3s orbital contains one electron, therefore, neutral sodium atoms have one valence electron. Therefore, the number of valence electrons in O 2- is 8. The last number is how we know the number of valence electrons. When a compound dissolves in a liquid, the compound separates into ions that distribute themselves evenly throughout the liquid. The same goes for every other element listed, as well. We write this as 2. Name the valence shell of this atom. Chlorine has 17 electrons, two in its innermost shell, eight in the next shell and seven in the third subshells that hold up to eight electrons. Sodium, with a total of 11 electrons, has only one electron in its third and outermost shell. For example, in the same row as sodium, the element in the next-to-last column is chlorine. How many valence electrons are in an atom of chlorine? Updated on: Oct For the so-called transition metals, valence electrons are not as well defined.
In the study of chemical reactivity, we will find that the electrons in the outermost principal energy level are very important and so they are given a special name. Valence electrons are the electrons in the highest occupied principal energy level of an atom. Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron.
What valence electrons are located in 4s24p3? While the elements with one valence electron are located on the left of the periodic table, the elements that need one valence electron to complete their outermost shells are found in the second to last column. The electronic configuration is 2 , 8. This valence electron is located in sodium's third energy shell. Impact of this question views around the world. How many valence electrons does hydrogen have? Your tool of choice here will be the Periodic Table of Elements. Explain with the help of two examples of different ions. The easiest way is to use the periodic table as your guide. Furthermore, every atom on the periodic table below lithium Li will also have one valence electron i. The second "shell" can have up to 8 electrons. Therefore, the number of valence electrons in O 2- is 8. Therefore, the number of valence electrons in sodium ion is 8. Print Page Previous Next.

Curious question
It agree, very useful message
It is remarkable, this valuable opinion