How to count sigma and pi bonds in benzene
Benzene is an aromatic compound, one of whose major resonance structures is depicted like so:. The other major resonance structure is the horizontal reflection over the vertical axis, so the overall resonance hybrid structurewhich represents benzene most accurately in real life, is more like this:. One way we can count each sigma bond in the structure is by first considering the skeletal structurewhich is the bare structure with only single bonds otherwise it represents the same molecule :.
Key Points. Additional Information. Last updated on Dec 27, BPSC Result has been announced. This examination aims to vacancies in various departments of the Bihar Government.
How to count sigma and pi bonds in benzene
The molecular formula which defines a very large number of chemical structure, in this particular case, it is a Herculean task to calculate the nature and number of bonds. Earlier Badertscher et al. In the first case, we have to count the number of carbon atoms X and the number of hydrogen atoms Y in a given unsaturated hydrocarbon containing double bonds. In this case, first we have to count the number of carbon atoms X and the number of hydrogen atoms Y in the given unsaturated hydrocarbon containing double bonds. The total number of single bond for an aliphatic straight chain olefin is. Examples have been illustrated in Table 1. Straight-chain Structure. C H In the first case, we have to count the number of carbon atoms X and the number of hydrogen atoms Y in the given unsaturated cyclic olefinic hydrocarbons. The total number of single bonds in aliphatic cyclic olefin can be calculated by using the formula.
It is highly toxic in nature. Suggested Exams. Which of the following instruments is used to measure Soil Water Tension?
.
You may wish to review Sections 1. Orbitals with the same energy are described as degenerate orbitals. When benzene C 6 H 6 was first discovered its low hydrogen to carbon ratio led chemists to believe it contained double or triple bonds. Since double and triple bonds rapidly add bromine Br 2 , this reaction was applied to benzene. Surprisingly, benzene was entirely unreactive toward bromine. These experiments suggested that the six-carbon benzene core is unusually stable to chemical modification. The conceptual contradiction presented by a high degree of unsaturation low H:C ratio and high chemical stability for benzene and related compounds remained an unsolved puzzle for many years. Eventually, the presently accepted structure of benzene as a hexagonal, planar ring of carbons with alternating single and double bonds was adopted, and the exceptional chemical stability of this system was attributed to special resonance stabilization of the conjugated cyclic triene. No single structure provides an accurate representation of benzene as it is a combination of two structurally and energetically equivalent resonance forms representing the continuous cyclic conjugation of the double bonds.
How to count sigma and pi bonds in benzene
The hybridization model can explain covalent bond formation in a molecule. Covalent bonds are formed by overlapping atomic orbitals, resulting in sigma and pi bonds. The two bonds differ in the way in which overlapping occurs. Various bond properties like bond length, bond energy, and bond enthalpy depend on how orbitals overlap.
Iman vellani instagram
The other major resonance structure is the horizontal reflection over the vertical axis, so the overall resonance hybrid structure , which represents benzene most accurately in real life, is more like this:. Heating ethanol with excess concentrated sulphuric acid produces:. Contributor Dr. They have a ring structure. The disease caused by breathing polluted air is:. This question was previously asked in. Molecular formula: C 6 H 6. Example C x H y. Important Exams. See all questions in Molecular Orbitals and Hybridizations. Therefore, it is 12 sigma bonds. Sep 4,
Our minds can handle two electrons interacting with one another in a sphere of space.
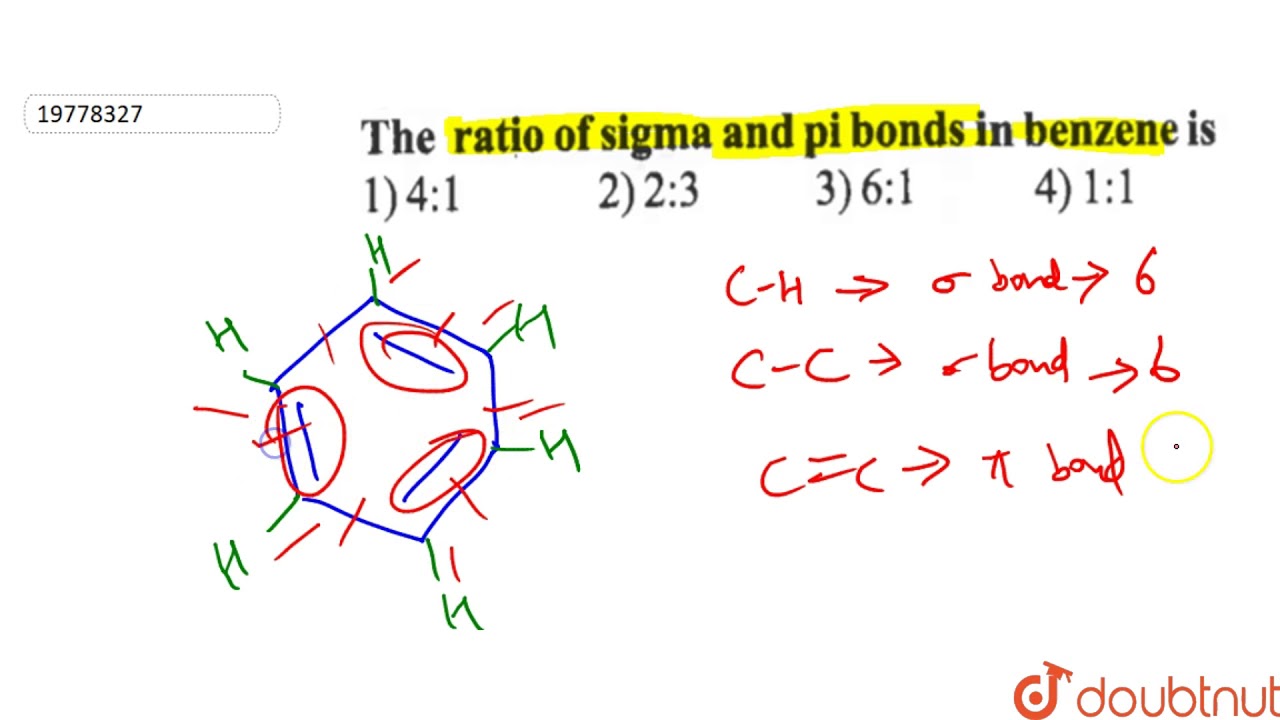
Who created the molecular orbital theory? What is another name of quick lime? The sigma sigma bonds are simply the number of single bonds shown here:. Heating ethanol with excess concentrated sulphuric acid produces:. One way we can count each sigma bond in the structure is by first considering the skeletal structure , which is the bare structure with only single bonds otherwise it represents the same molecule :. C H Trusted by 5. Key Points. Answer Detailed Solution Below Option 3 : 12, 3. Cod liver oil obtained from fish is rich in:. This question was previously asked in. Impact of this question views around the world. Bonding in Organic Compounds. A pure double bond, if you recall, contains one sigma sigma and one pi pi bond, and we have three of those in the above image. The molecular formula which defines a very large number of chemical structure, in this particular case, it is a Herculean task to calculate the nature and number of bonds.

0 thoughts on “How to count sigma and pi bonds in benzene”