Hsp70
Federal government websites often end in. The site is secure. Hsp70 proteins are central components of the cellular network of molecular chaperones and folding catalysts. They assist a large variety of hsp70 folding processes in the cell by transient association hsp70 their substrate binding domain with short hydrophobic peptide segments within their substrate proteins, hsp70, hsp70.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The kDa heat shock proteins Hsp70s are ubiquitous molecular chaperones that act in a large variety of cellular protein folding and remodelling processes. They function virtually at all stages of the life of proteins from synthesis to degradation and are thus crucial for maintaining protein homeostasis, with direct implications for human health.
Hsp70
Federal government websites often end in. The site is secure. Heat shock protein 70 Hsp70 is a molecular chaperone that is expressed in response to stress. In this role, Hsp70 binds to its protein substrates and stabilize them against denaturation or aggregation until conditions improve. This review provides a brief review of Hsp70 structure and function and then explores some of the emerging opportunities and challenges for drug discovery. Some of the functions of the cytosolic isoforms, Hsc70 and Hsp72, are thought to be redundant, but the transcription of Hsp72 is highly responsive to stress and Hsc70 is constitutively expressed. In the ER and mitochondria, the Hsp70 family members are thought to fulfill specific functions and have unique substrates, with BiP playing key roles in the folding and quality control of ER proteins and mtHsp70 being involved in the import and export of proteins from the mitochondria. For the purposes of this review, we will often use Hsp70 as a generic term to encompass the shared properties of the family members. The Ia and IIa subdomains interact with ATP through a nucleotide-binding cassette related to those of hexokinase, actin and glycerol kinase. For example, Hsp70 is known to interact with clathrin, components of the transcriptional activation complex, nuclear hormone receptors and many others. The SBD contains a beta-sandwich and a helical lid domain. B Schematic of ATP hydrolysis and the role of co-chaperones. Hsp70 binds tightly to ATP, with some reports of E. Thus, in the presence of a J-protein, the release of ADP becomes the rate-limiting step.
Leone G, hsp70. As the field of Hsp70 inhibitors matures, increasing utilization of structural analysis and broader assay profiling will ultimately accelerate discovery.
Center on AT3G Full-screen view. Locus: AT3G What's new on this page. Data Source. Powered by BAR Webservices. AT1G Hsp70b ,. AT4G cpHsc ,.
Heat shock protein 70 HSP70 is activated under stress response. Its involvement in cell protection, including energy metabolism and quality control makes it a promising pharmacological target. However, cell permeability and functionality of these exogenously applied proteins inside the cells is still disputable. Here, using fluorescence- labeled HSP70, we have studied permeability and distribution of HSP70 inside primary neurons and astrocytes, and how exogenous HSP70 changes mitochondrial metabolism and mitophagy. We have found that exogenous recombinant HSP70 can penetrate the neurons and astrocytes and distributes in mitochondria, lysosomes and in lesser degree in the endoplasmic reticulum. Increased mitochondrial membrane potential was associated with higher mitochondrial ROS production and activation of mitophagy.
Hsp70
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The metazoan kDa heat shock protein HSP70 family contains several members localized in different subcellular compartments. Here we provide novel insights into the evolution of these important molecular chaperones. Phylogenetic analyses of full-length HSP70s from a broad range of phyla revealed an ancient duplication that gave rise to two lineages from which all metazoan cytosolic HSP70s descend. One lineage A contains a relatively small number of genes from many invertebrate phyla, none of which have been shown to be constitutively expressed i. The other lineage B included both inducible and constitutive genes from diverse phyla. Consistent with the diversification history within each group, inducible members show lower purifying selection pressure compared to constitutive members.
Twitch xxx
Chemical structure of a representative sulfoglycolipid adaSGC. Goloubinoff P. Nonenzymatic posttranslational protein modifications in ageing. The roles of the two zinc binding sites in DnaJ. In , she entered the Chemical Biology Ph. Forms complex heat shock granule with J3. Bonini N. April CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation. They assist a large variety of protein folding processes in the cell by transient association of their substrate binding domain with short hydrophobic peptide segments within their substrate proteins. Hspdependent protein folding in vitro occurs typically on the time scale of minutes or longer.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer.
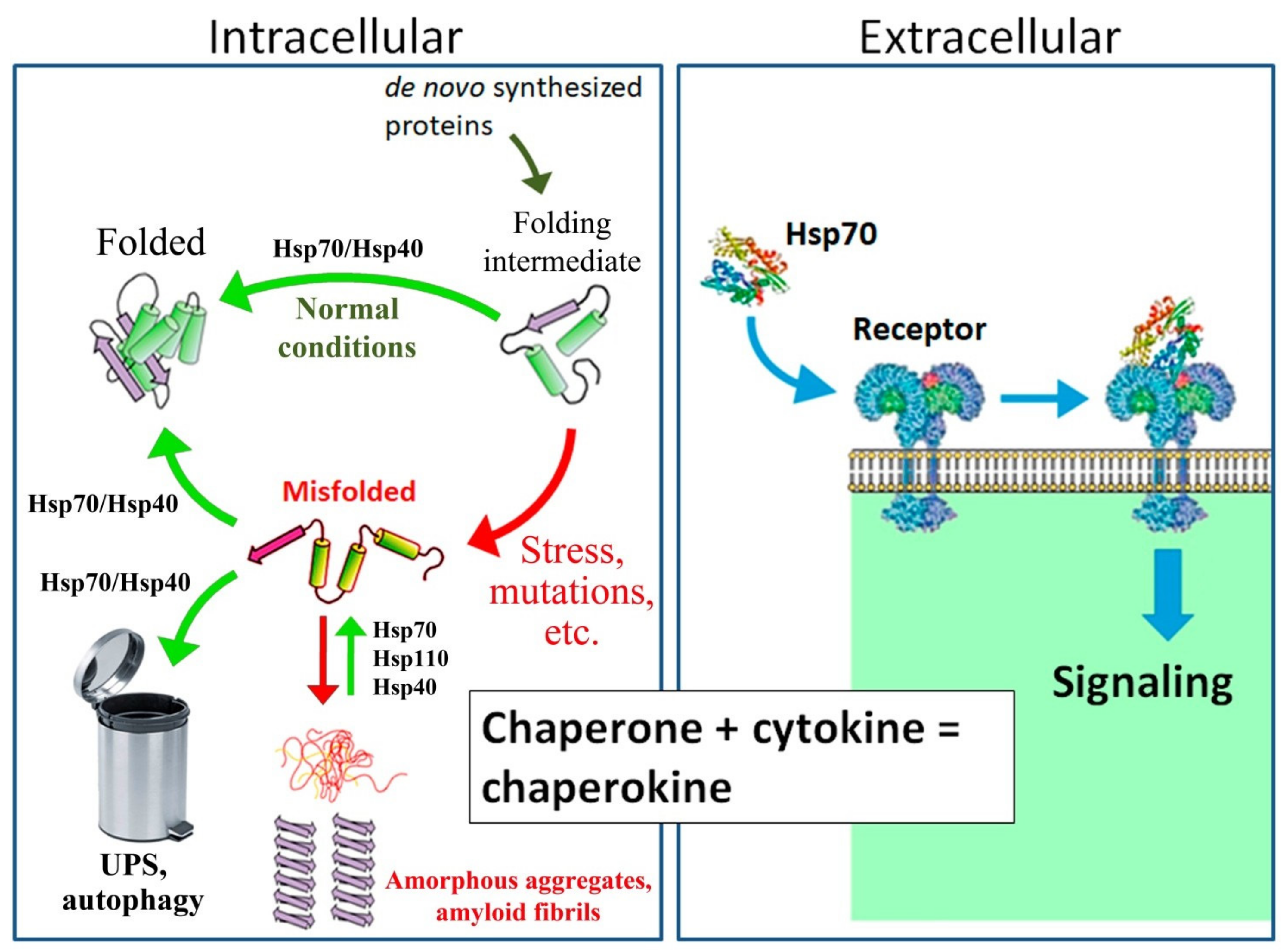
Protein Sci. Schlecht, R. Baaklini, I. The differences between Hsp70 homologs in nucleotide dissociation go along with differences in the regulation of this step. Article PubMed Google Scholar. Since the average protein size in eukaryotic cells is increased 52 kDa in humans as compared to bacteria 35 kDa in E. Celliti et al. In this way stress response and apoptosis are linked to each other. Moran Luengo, T. Chaperokine Activity of Heat Shock Proteins.

I can consult you on this question. Together we can come to a right answer.
Big to you thanks for the help in this question. I did not know it.
In my opinion you commit an error. Let's discuss. Write to me in PM, we will communicate.