Hydrogen bromide lewis dot structure
Wiki User. HBr has a dipole.
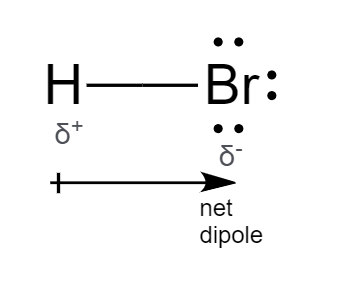
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom. When we draw a lewis structure, there are several steps to follow. Number of steps can be changed according the complexity of the molecule or ion.
Hydrogen bromide lewis dot structure
Wiki User. The electron dot formula for a monoatomic hydrogen is H. However, elemental hydrogen is diatomic, so most hydrogen atoms would be found as H:H. Please note the parentheses above are for clarification and are not part of the electron dot diagram. Lewis structure, electron dot diagram, electron dot structure WikiAnswers does not display diagrams, look in the related link below. The dot and cross diagram, or Lewis structure, for hydrogen bromide is as follows: Place a Br atom in the center and single bond it to one H atom. The Br atom then has 3 lone pairs placed around it. Yes, in a Lewis diagram, the valence electrons are shown by dots around them. Louis Dot created the Dot Diagram. The electrons in the outermost shell of an atom is known as the valence electrons. These valence electrons determine the reactivity of the atom.
There are two elements in hydrogen bromide; hydrogen and bromine. The electrons in the outermost shell of an atom is known as the valence electrons. What is the system used to represent the valence electrons around the chemical symbol of an element?
.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons.
Hydrogen bromide lewis dot structure
Ready to learn how to draw the lewis structure of HBr? Here, I have explained 6 simple steps to draw the lewis dot structure of HBr along with images. The Bromine atom has 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me.
Multiply fractions online
Because HBr molecule is a simple molecule and there is no overall charge, all of these steps are not required to use to complete the lewis structure. For, HBr, Total pairs of electrons are four in their valence shells. The electrons in the outermost shell of an atom is known as the valence electrons. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom. The Lewis dot structure for hydrogen bromide HBr consists of a single covalent bond between the hydrogen atom and the bromine atom. Because there are no charges on atoms, no need to worry about reducing charges as a step of obtaining the best lewis structure. Trending Questions. How do you draw the Lewis dot diagram for hydrogen chloride? There are two elements in hydrogen bromide; hydrogen and bromine. Trending Questions. Is HBr a dipole dipole? What is the Lewis dot diagram for c2h7n?
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule.
These valence electrons determine the reactivity of the atom. More answers. Q: Lewis dot diagram of hydrogen Write your answer However those all steps are mentioned and explained in detail in this tutorial for your knowledge. What is the symbolic notation for hydrogen? Previously Viewed. Previously Viewed. What is the Lewis dot diagram for calcium and fluorine? WikiAnswers does not display diagrams, look in the related link below. Resources Leaderboard All Tags Unanswered. The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. The electron dot diagram of uranium is:. HBr has a dipole.

I apologise, but, in my opinion, you are not right. I am assured.