Hydrogen sulfide polar or nonpolar
Hydrogen sulfide is a colorless molecule with a chemical formula H 2 S. It is poisonous and has a foul odor like a rotten egg. The compound hydrogen sulphide has the chemical formula H 2 S. Hydrogen sulphide is a covalent molecule made up of two hydrogen atoms bound to a sulphur atom in the middle.
H 2 S is the chemical formula for the compound hydrogen sulfide. Hydrogen sulfide is a covalent compound that is composed out of 2 hydrogen atoms bonded to a central sulfur atom. Like water H 2 0 , hydrogen sulfide is a hydrogen chalcogenide—a compound made from hydrogen and a group 16 element oxygen, sulfur, selenium, tellurium. Hydrogen sulfide is non-polar on account of its nonpolar H—S bonds. The EN difference between hydrogen and sulfur is 0.
Hydrogen sulfide polar or nonpolar
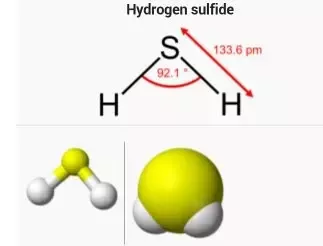
To determine if H 2 S hydrogen sulfide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The remaining four electrons go to the sulfur:. The central atom has a steric number of 4 — two atoms and two lone pairs. The electron geometry, therefore, is tetrahedral , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-H bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. Although sulfur is not as electronegative as oxygen, just like in water the dipoles are pointing from the hydrogen to the more electronegative atom, and the overall molecular dipole is pointing up as drawn:. Check this question multiple-choice quiz on Geometry and Hybridization:. Notify me of followup comments via e-mail.
Thus, hydrogen hydrogen sulfide polar or nonpolar is one of the main constituents of the sulfur cycle. The central atom has a steric number of 4 — two atoms and two lone pairs. Hydrogen sulfide is a covalent compound that is composed out of 2 hydrogen atoms bonded to a central sulfur atom.
.
Hydrogen Sulfide is a common chemical compound that is useful for analyzing inorganic compounds of metal ions. It has the chemical formula of H 2 S. The molecule has two Hydrogen atoms and a single Sulfur atom. H 2 S is also a precursor for elemental Sulfur. It also plays a vital role in signaling pathways in the human body.
Hydrogen sulfide polar or nonpolar
Hydrogen sulfide is a colorless molecule with a chemical formula H2S. It is poisonous and has a foul odor like a rotten egg. So, is H2S polar or nonpolar? H2S is a slightly polar molecule because of its bent shaped geometrical structure and the small difference between the electronegativity of Hydrogen 2. It was discovered in the year by a chemist named Carl Wilhelm Scheele. This gas is produced by human bodies and we uses it as a signaling molecule. Polarity is described as how electrons are distributed in the molecule. It shows wherewith electrons are attracted and pulled by the most electronegative atom.
Harley quinn r 34
The human body can tolerate low concentrations of hydrogen sulfide for some time. Ask us Now! It is used for manufacturing pesticides for crops on a larger scale. If the difference is greater than 2, then the bond is completely polar, and is more properly referred to as an ionic bond. For instance, some bacteria that operate in the absence of oxygen use sulfate ions SO 4 — as the terminal electron acceptor during cellular respiration which reduces it into H 2 S. It is poisonous and has a foul odor like a rotten egg. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. Post your question. Our panel of experts willanswer your queries. If two elements have an EN difference between 0.
H2S is a slightly polar molecule because of the small difference in electronegativity values of Hydrogen 2. In addition, the presence of two lone pairs that are on the opposite side of the two Hydrogen atoms also makes the molec ule more polar and causes a bent shape geometrical structure of H 2 S.
The remaining four electrons go to the sulfur: The central atom has a steric number of 4 — two atoms and two lone pairs. Hydrogen sulphide is a covalent molecule made up of two hydrogen atoms bound to a sulphur atom in the middle. Related articles Related Qustion. Hydrogen is a partial positive charge as it is now left with fewer positive charges. Similar mechanisms also result in the formation of hydrogen sulfide in thermal ocean vents. It directs towards more electronegative atom. Hydrogen Sulfide is a colorless, very poisonous, flammable gas with the characteristic foul odor of rotten eggs. The activity of sulfidogenic bacteria and their hydrogen sulfide products are responsible for the rotting smell associated with places with large quantities of decaying organic matter, like marshes or sewers. If the difference in electronegativity is less than 0. To determine if H 2 S hydrogen sulfide is polar or nonpolar, we need to first determine its geometry. Being a corrosive, it destructs metals like copper turning into green in color after the reaction. Mar 7, How many neutrons, protons and electrons does a sodium atom have? Hydrogen sulfide is a triatomic 3-atom molecule that consists of a central sulfur atom and 2 terminal hydrogen atoms.

Excuse, that I interfere, would like to offer other decision.
Willingly I accept. The question is interesting, I too will take part in discussion. Together we can come to a right answer.