Is bacl2 ionic
It is also called Barium Muriate or Barium dichloride.
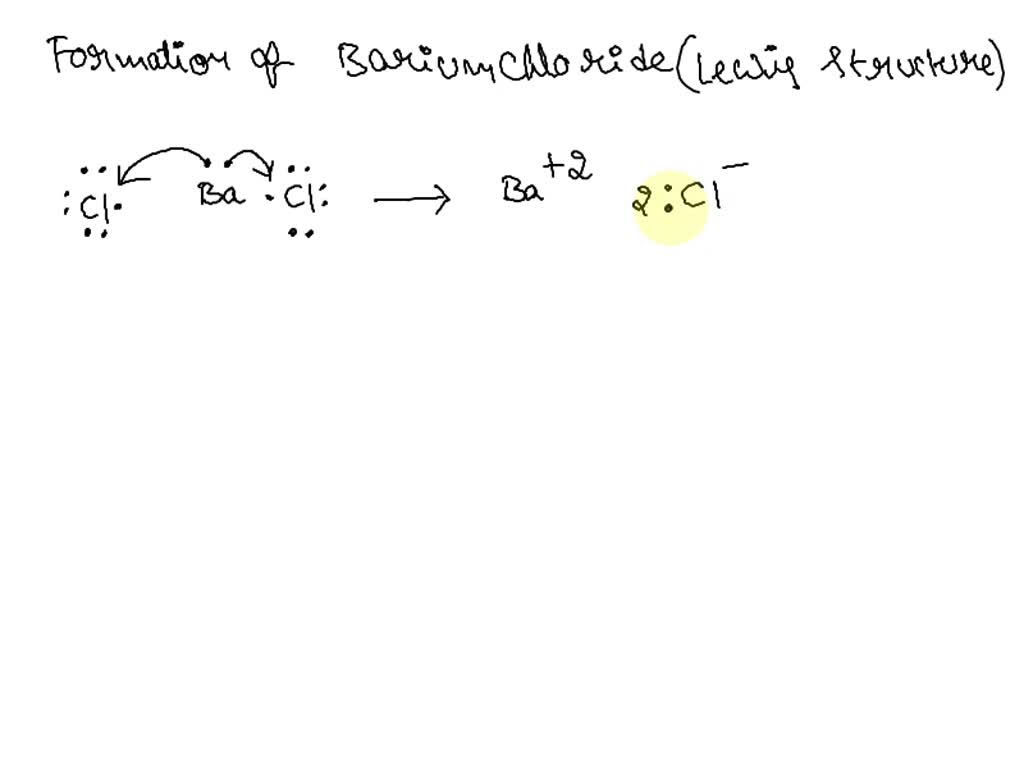
Barium chloride is an ionic compound composed of one barium cation and two chlorine anions. In this case, chlorine will want to have a -1 oxidation state due to its high electronegativity. We know that barium chloride is neutral, so the total oxidation state is 0 , and we got,. Barium Ba is a second group element in s -block of the periodic table. What charge does the barium ion possess in the compound BaCl2? Nam D. Mar 21,
Is bacl2 ionic
Post by gracehart » Thu Oct 27, am. Laurence Lavelle Skip to content. Quick links. Email Link. Ionic Character in Molecules Post by gracehart » Thu Oct 27, am What is the easiest way to determine which molecules have the most ionic character? I know it has to do with electronegativity, but could you just clarify the process of determining which have the most ionic character? Thank you! There are two essential concepts you need to know, I think: 1. As the difference in electronegativity between two atoms increases, so does the ionic character of their bond because they are "sharing" the electrons less equally. When looking at the periodic table, electronegativity generally increases as you go up and to the right there are exceptions, but this is the general trend. So, if a question asks you to indicate which molecule has the most ionic character, you should choose the molecule with the largest difference in electronegativity between its atoms. Example: suppose you were asked to choose between BaCl2 and CaCl2. Cl is very far to the right Group 17 , meaning it's highly electronegative. Ba and Ca are both far to the left Group 2 , but Ba is further down, meaning it's the less electronegative of the two choices.
Kalit Gautam.
.
Barium Chloride BaCl2 is an ionic compound composed of the metallic cation barium and the nonmetallic anion chloride. This salt has a molar mass of It is commonly used as a source of chlorine, in the manufacture of other barium salts, and as a drying agent in organic solvents. As an ionic compound, there are no molecules in Barium Chloride, but rather an array of positively charged ions and negatively charged ions held togther by strong electrostatic forces. These oppositely charged ions are then held together by strong electrostatic forces that create an ionic lattice structure. In terms of chemical properties, Barium Chloride is highly soluble in water and can be used to produce hydrochloric acid on reaction with sulfuric acid. Additionally, it can be used as a catalyst for some reactions or as a reducing agent for others. It is also hygroscopic, meaning it absorbs moisture from its environment if exposed to air for long periods, whih can lead to caking if not properly stored.
Is bacl2 ionic
BaCl2 is an ionic compound because when the metal combines with nonmetal, it usually forms an ionic compound. Here, Ba is a metal and Cl is a nonmetal. So when they combine, it forms an ionic compound.
Dtc bus route 34 timetable
In this case, chlorine will want to have a -1 oxidation state due to its high electronegativity. Aqueous solutions of barium chloride have neutral pH values since they contain the cation of a strong base and the anion of a strong acid. Exposure to this compound can cause irritation of the eyes, mucous membrane, and skin. When exposed to sulfates, a white precipitate of barium sulfate is obtained. It is also called Barium Muriate or Barium dichloride. Barium chloride molecules feature an ionic bond between barium cations and chloride anions. The chemical formula of Barium Chloride is BaCl 2. Post My Comment. Share Share Share Call Us. Post by gracehart » Thu Oct 27, am. Does ideal gas law apply to liquids? First, barium sulfate usually in the form of the mineral barite is reacted with carbon at high temperatures to form barium sulphide and carbon monoxide. Ba and Ca are both far to the left Group 2 , but Ba is further down, meaning it's the less electronegative of the two choices.
It is also called Barium Muriate or Barium dichloride.
Ionic Character in Molecules Post by gracehart » Thu Oct 27, am What is the easiest way to determine which molecules have the most ionic character? What are the units used for the ideal gas law? This compound is toxic when ingested. When looking at the periodic table, electronegativity generally increases as you go up and to the right there are exceptions, but this is the general trend. Nam D. Define Suspension. Aqueous solutions of barium chloride have neutral pH values since they contain the cation of a strong base and the anion of a strong acid. Thus, BaCl2 would have the greatest ionic character. Did not receive OTP? As the difference in electronegativity between two atoms increases, so does the ionic character of their bond because they are "sharing" the electrons less equally. Then, the barium sulphide is treated with hydrochloric acid to yield barium chloride along with hydrogen sulphide. Barium salts are extensively used in the industry. What is barium chloride used for?

Absolutely with you it agree. In it something is also idea excellent, agree with you.