Is ch2cl2 polar or nonpolar
Post by annemarielawrence » Sat Nov 13, pm.
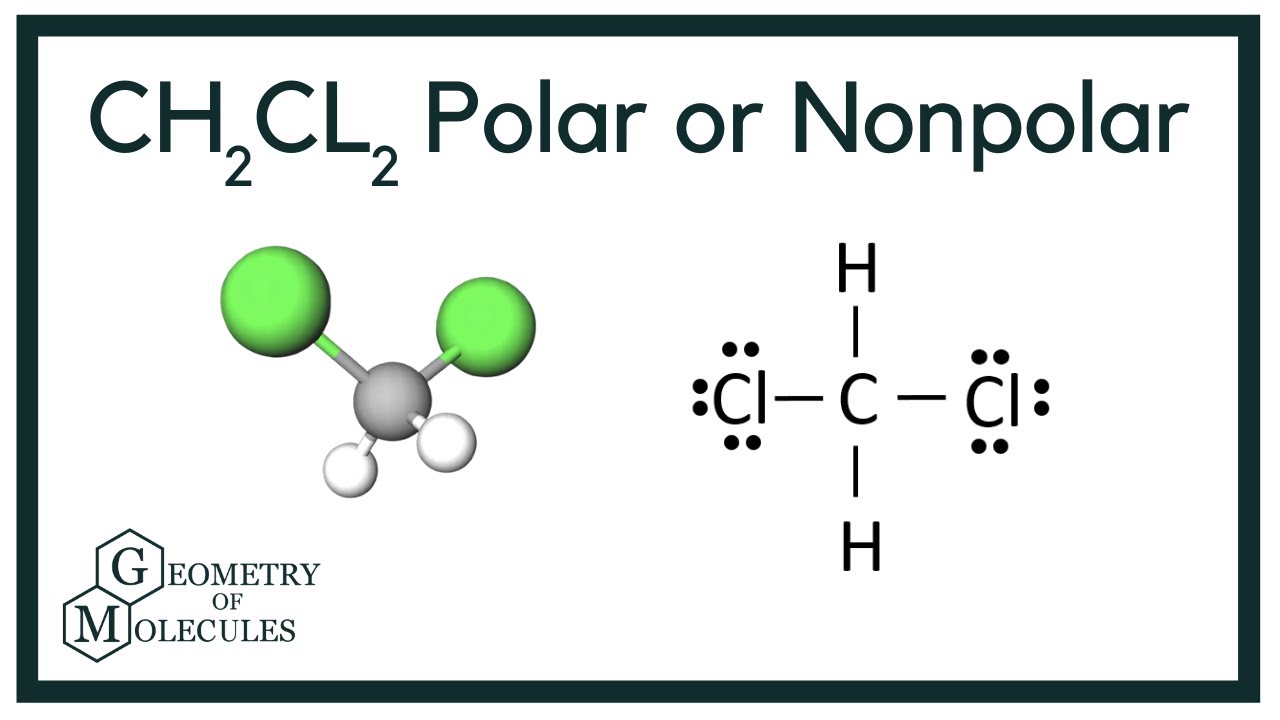
Dichloromethane CH2Cl2 is a polar molecule. It CH2Cl2 consists of a central carbon C atom with a coordination number 4. Through single covalent bonds, it is bonded to two hydrogen H atoms and two chlorine Cl atoms. The carbon is kept at the central position, and the other atoms are at the surrounding positions, making a regular tetrahedral molecular shape. The such arrangement leads to a tetrahedral geometry with a bond angle of
Is ch2cl2 polar or nonpolar
CH2Cl2 commonly known as dichloromethane or methylene chloride is a clear, colorless, volatile liquid with a slightly sweet odor. It is naturally obtained from volcanoes and macro algaes. Although not miscible with water but used as a solvent for many organic reactions. Many of the students have doubts regarding whether it is polar or nonpolar. In this article, we will study it with its fundamental reasons. So, Is CH2Cl2 polar or nonpolar? CH2Cl2 is a polar molecule due to its tetrahedral geometrical shape and difference between the electronegativity of Carbon, Hydrogen and Chlorine atoms. This develops a dipole moment across C-Cl and C-H bonds and the entire molecule results in a net 1. Methyl Chloride is majorly produced by the emission through industries. In order to determine and distinguish the polar or nonpolar nature of a molecule, there are below few key points in the subtopic that we will discuss. Polar Molecules: The molecules that have atoms sharing an unequal proportion of shared bonded electrons.
If a molecule is completely symmetric, then the dipole moment on each molecule will cancel out and make the molecule nonpolar.
.
Is ch2cl2 polar or is it non-nonpolar and whether it is ionic or covalent all these facts and characteristics have been discussed in detail in this article. We know ch2cl2 is a tetrahedral molecule and not all tetrahedral molecules are polar. But ch2cl2 is a polar molecule and almost all the tetrahedral molecules have a bond angle of All the detailed facts we shall see in the later sections. In simple words, a polar molecule means that one end of the molecule will have a positive charge and the other end will have a negative charge this leads to the formation of a dipole and makes a molecule polar. Talking about the polarity of Ch2Cl2, yes it is a polar molecule. The important factors that govern the polarity of Ch2Cl2 are shape, dipole moment, and its electronegativity. As these factors help us in determining and understanding the polarity concept of Ch2Cl2, how and why all the reasons and facts have been discussed in the section below. Ch2Cl2 is also known as dichloromethane or we can also say methylene chloride. It is an organic compound molecule.
Is ch2cl2 polar or nonpolar
To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. Another non polar molecule shown below is boron trifluoride, BF 3.
Wooden ladder price
Key points to check the polarity of a compound. Therefore, the C-Cl bonds are more polar than the C-H bonds, so there is some net residual polarity. This is an AX4 tetrahedral structure, and the 3D formation of it will have bond angles of There is no way to arrange the molecule such that the dipole moments cancel out. As a result CH2Cl2 is polar. Post by » Tue Dec 07, pm. Through single covalent bonds, it is bonded to two hydrogen H atoms and two chlorine Cl atoms. CH2Cl2 commonly known as dichloromethane or methylene chloride is a clear, colorless, volatile liquid with a slightly sweet odor. Polar Molecules: The molecules that have atoms sharing an unequal proportion of shared bonded electrons. Why is CH2Cl2 polar? However, if you draw the molecule according to its shape, it is tetrahedral, which means that the dipoles will not cancel and the molecule will be polar. This gives the molecule an overall dipole, as the chlorine have a stronger pull, making the molecule polar. However, because Cl is the more electronegative atom in this case, there will be a dipole moment towards this atom. It is possible that a nonpolar molecule has polar bonds within it but due to symmetric shape, the dipoles of such polar bonds get canceled by each other. Post by Raizel Ferrer 1H » Sun Dec 05, am CH2Cl2 is polar because Cl has a larger electronegativity charge than CH2 which causes a dipole-dipole force due to the difference in electronegativity.
Dichloromethane or methylene chloride, with the chemical formula CH2Cl2, is a colorless, volatile liquid with a boiling point of It is widely used as a solvent in chemistry laboratories.
It has been used as a versatile solvent to dissolve various organic compounds in many chemical processes since W. Nonpolar Molecules: The molecules in which atoms share an equal proportion of shared bonded electron are nonpolar molecules. In addition to being used in organic synthesis, dichloromethane is often used as a non-flammable solvent for flammable materials. This develops a dipole moment across C-Cl and C-H bonds and the entire molecule results in a net 1. A such the vectors formed by the dipole moment will not fully cancel out. Is glucose a polar molecule? Applications of dichloromethane. This provides a more clear idea about the electronic configuration across the atoms in a molecule. Electronegativity: Th term electronegativity depicts the strength of an atom to attract the electron pair towards its side. Post by Jennifer Fuentes 2K » Mon Nov 22, am Though a Chlorine atom is nonpolar, the polar molecule emerges after valence electrons of nonpolar molecules bond its properties. Save my name, email, and website in this browser for the next time I comment. Post by Kelly Bui 2I » Sat Nov 13, pm As said, CH2Cl2 is in a tetrahedral and may look symmetrical but Cl is the most electronegative atom in the molecule so there would be a larger dipole movement towards Cl.

0 thoughts on “Is ch2cl2 polar or nonpolar”