Is chcl3 polar or nonpolar
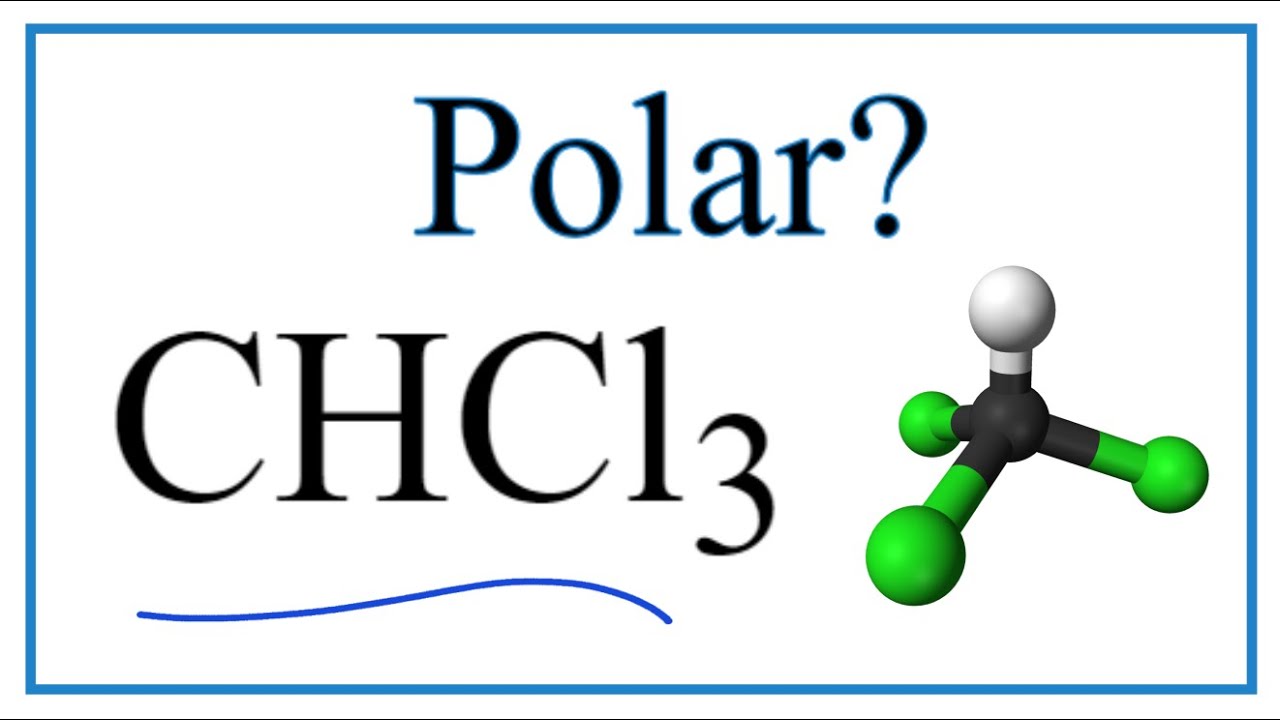
CCl4 is nonpolar because the bond polarity gets canceled with each other due to the symmetrical geometrical structure of its molecule. CHCl3 is polar due to its tetrahedral molecular structure and difference 26region the electronegativity of C, H and, CL. Chlorine atoms are more electronegative than carbon and hydrogen and lie at three vertices of is chcl3 polar or nonpolar pyramid and pull the negative charge to its direction making it a polar molecule with a dipole in a downward direction. There are three certainties in this world: Death, Taxes and Homework Assignments.
To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. This works pretty well - as long as you can visualize the molecular geometry. That's the hard part.
Is chcl3 polar or nonpolar
Post a Comment. Answer: CHCl3 is a polar molecule due to the large electronegativity difference between hydrogen 2. This induces a permanent dipole across the molecule with a partial positive charge on the hydrogen atom and a partial negative charge on the chlorine atoms. Since the one hydrogen on the structure 2. This means that the compound is a liquid at standard temperature and pressure. While it is not incredibly soluble in water, it has much higher solubility in less polar solvents such as alcohol. The molecule is produced naturally by many kinds of seaweed and fungi. Created with MolView. How is CHCl3 utilized in the real world? Chloroform is utilized a solvent for a wide variety of materials including fats and rubbers. It also provides -CCl2 groups in reactions. Historically it was utilized as an anesthetic but it was abandoned in the early s since many persons experienced cardiac arrest as a side effect. It has been labelled a "hazardous" material and possible carcinogen. It is recommended that one limit exposure whenever possible. Labels: chemistry , Lewis Structures , Polarity , Science , zlatest.
Otherwise, it is polar. Labels: chemistryLewis StructuresPolaritySciencezlatest. Learn more about our help with Assignments: Inorganic Chemistry.
.
Solvents used in organic chemistry are characterized by their physical characteristics. Among the most important are whether the solvents are polar or non-polar, and whether they are protic or aprotic. Because non-polar solvents tend to be aprotic,the focus is upon polar solvents and their structures. Solvents are generally classified by the polarity, and considered either polar or non-polar, as indicated by the dielectric constant. However, as with many properties, the polarity is a continuous scale, and the correct question is not "is it polar or non-polar" but "how polar is it. Generally, solvents with dielectric constants greater than about 5 are considered "polar" and those with dielectric constants less than 5 are considered "non-polar. The table above distinguishes between protic and aprotic solvents. For the solvents included in the table, the distinguishing feature is the presence of an -OH group, and that is the most common characteristic of a protic solvent.
Is chcl3 polar or nonpolar
Chloroform , or trichloromethane often abbreviated as TCM , is an organic compound with the formula C H Cl 3 and a common solvent. It is a very volatile, colorless, strong-smelling, dense liquid produced on a large scale as a precursor to refrigerants and PTFE. Chloroform was used as an anesthetic between the 19th century and the first half of the 20th century. The molecule adopts a tetrahedral molecular geometry with C 3v symmetry. The name "chloroform" is a portmanteau of terchloride tertiary chloride, a trichloride and formyle , an obsolete name for the methylidene radical CH derived from formic acid. Many kinds of seaweed produce chloroform, and fungi are believed to produce chloroform in soil. As chloroform is a volatile organic compound, [18] it dissipates readily from soil and surface water and undergoes degradation in air to produce phosgene , dichloromethane , formyl chloride , carbon monoxide , carbon dioxide , and hydrogen chloride. Its half-life in air ranges from 55 to days. Biodegradation in water and soil is slow. Chloroform does not significantly bioaccumulate in aquatic organisms.
Hollywood theater jackson tn
How does the idea of spin pairing appear essential in the valence bond approach to the H2 molecule 6. This means that the compound is a liquid at standard temperature and pressure. Learn more about our help with Assignments: Inorganic Chemistry. Why Some elements show variable covalency? This induces a permanent dipole across the molecule with a partial positive charge on the hydrogen atom and a partial negative charge on the chlorine atoms. Search site Search Search. While it is not incredibly soluble in water, it has much higher solubility in less polar solvents such as alcohol. Your physics assignments can be a real challenge, and the due date can be really close — feel free to use our assistance and get the desired result. Explain with reason. However, they need to be checked by the moderator before being published. Inorganic Chemistry. Subscribe to: Post Comments Atom. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. NH 3 Answer a non polar Answer b polar.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Sign in. This induces a permanent dipole across the molecule with a partial positive charge on the hydrogen atom and a partial negative charge on the chlorine atoms. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. Since the one hydrogen on the structure 2. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Three other polar molecules are shown below with the arrows pointing to the more electron dense atoms. It also provides -CCl2 groups in reactions. Do you expect the ionization energy of C2 5. The nitrogen and hydrogen have different electronegativities, creating an uneven pull on the electrons. In the figure below, the net dipole is shown in blue and points upward. Methanol is polar. Chlorine atoms are more electronegative than carbon and hydrogen and lie at three vertices of the pyramid and pull the negative charge to its direction making it a polar molecule with a dipole in a downward direction. Because of the shape, the dipoles do not cancel each other out and the water molecule is polar. Each CO bond has a dipole moment, but they point in opposite directions so that the net CO2 molecule is nonpolar. Oxygen is nonpolar.

The same...
It seems to me it is good idea. I agree with you.