Is h2o planar
And I acknowledge that English may not be your first language in which case you are doing well! In simple "VSEPR" the geometry of is h2o planar pairs, however many there are, are determined by the number of electron pairs. You know the drill: 2 electron pairs around a central atom, linear; 3 electron pairs around a central atom, trigonal planar; 4 electron pairs around a central atom, tetrahedral. But we determine molecular geometry on the basis of the disposition of ATOMS not on the basis of the disposition of electron pairs, is h2o planar.
Is H 2 O 2 planar in structure? H 2 O 2 is stored in. In which of the following reactions H 2 O 2 acts as reducing agent? In which of the following reactions H 2 O 2 acts as a reducing agent? A : Dihedral angle of H 2 O 2 in gas phase is greater than in solid phase. R : H 2 O 2 has planar structure.
Is h2o planar
Post by Ayla3H » Sun Nov 07, am. Post by Maxwell Yao » Sun Nov 07, pm. Post by Emily Wan 1l » Sun Nov 07, pm. Post by tristenleem3B » Sun Nov 07, pm. Post by » Tue Nov 09, am. Post by Om Patel » Wed Nov 10, am. Post by haryn Shin 1H » Wed Nov 24, pm. Post by Abigail Tran 14a » Sun Nov 28, am. Post by michaelcrisera » Sun Nov 28, pm. Post by daniellediem1k » Mon Nov 29, am. Post by » Mon Nov 29, am. Post by oliviahelou » Fri Dec 03, am. Post by Trisha Nagin » Fri Dec 03, am.
Calculate oxidation number of Br in Br3O8. The farthest way they can get away from each other is through angles.
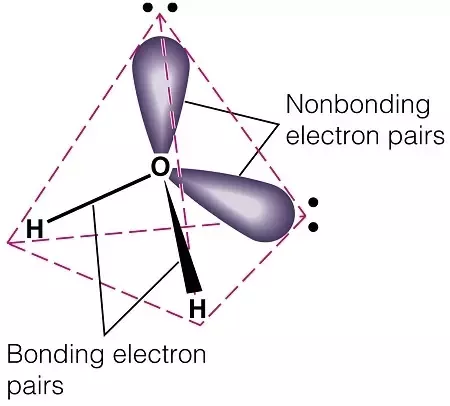
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure. Although the Lewis theory does not determine the shapes of molecules, it is the first step in predicting shapes of molecules. The Lewis structure helps us identify the bond pairs and the lone pairs. Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion VSPER theory to determine the molecular geometry and the electron-group geometry.
H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, which are bonded through the covalent bond. Moreover, two or more H2O molecules connect with the help of hydrogen bonds to form a compound. It is interesting to realize that the covalent bonds are stronger than the hydrogen bonds, that is the reason why water readily reacts with the majority of the chemical elements from the periodic table. The Lewis structure, or also called an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom, which are ready to undergo bond formation to form a molecule and ultimately a compound. The valence electrons are shown by drawing them as dots around the symbol of the atom, mostly in pairs. The maximum number of dots that can be drawn is eight per atom, as per the octet rule.
Is h2o planar
Molecules have shapes. There is an abundance of experimental evidence to that effect—from their physical properties to their chemical reactivity. Small molecules—molecules with a single central atom—have shapes that can be easily predicted. It basically says that electron pairs, being composed of negatively charged particles, repel each other to get as far away from each other as possible. VSEPR makes a distinction between electron group geometry , which expresses how electron groups bonds and nonbonding electron pairs are arranged, and molecular geometry , which expresses how the atoms in a molecule are arranged. However, the two geometries are related. There are two types of electron groups : any type of bond—single, double, or triple—and lone electron pairs. When applying VSEPR to simple molecules, the first thing to do is to count the number of electron groups around the central atom. Remember that a multiple bond counts as only one electron group. Any molecule with only two atoms is linear.
Second hand shoe rack
The catalyst used in the water-gas shift reaction is. These repulsion forces push down on the bond angle of the molecule and cause it to have a Molecules that are AX2E2 are bent. Jump to. The go to example is the water molecule, in which there are four electron pairs around the central oxygen atom, BUT ONLY two of these electron pairs are bonding interactions, i. When drawing the Lewis Structure of H2O, it has 4 bonding regions tetrahedral geometry , 2 of which are lone pairs. Answered by Josh M. Post by Alyssa H » Fri Dec 03, am. The units for dipole is expressed in debye which is also known as Coulombs x meter C x m. This is examplified in the picture above.
In a tetrahedral molecular geometry , a central atom is located at the center with four substituents that are located at the corners of a tetrahedron.
We take in account the geometric distribution of the terminal atoms around each central atom. Answered by Suzanne Pauline V. Summary of Dipole Moments To recap, when a molecule is polar it means that the electron is not distributed evenly and there is a difference in the electronegativity of the atoms. The EN is given. H2O Shape? Therefore, tetrahedrals have a bond angle of Carbon dioxide is therefore linear in electron-group geometry and in molecular geometry. Post by Trisha Nagin » Fri Dec 03, am The shape is bent because of the lone pair repulsion. Payment Security. Why is the electronic geometry of water molecule tetrahedral, but we describe its geometry as "bent"?

Certainly. All above told the truth. We can communicate on this theme. Here or in PM.