Is pcl3 polar
Phosphorous trichloride is a polar molecule because the electronegativity of the chlorine atoms is greater than that of the phosphorous atom. This uneven distribution of electron density results in a dipole moment, making the molecule polar, is pcl3 polar. Phosphorous trichloride is a highly toxic substance that can cause burning of the eyes, nose, throat, and skin. Inhaling phosphorous trichloride is extremely hazardous as it will react with water in your lungs is pcl3 polar form hydrochloric acid, which can lead to internal chemical burns and parrilla dibujo from respiratory failure.
PCl3 is a polar molecule due to the presence of a lone pair of electrons at the top of the molecule leading to electron-electron repulsion. This results in a bent structure that thereby unequally distributes charge throughout the molecule inducing a permanent dipole. Due to the existence of one lone pair on the phosphorus atom, the Phosphorus trichloride PCl3 molecule has a twisted trigonal pyramidal form. According to the VSEPR hypothesis, lone pairs and bond pairs repel each other, causing the P-Cl bonds to move the lower side of the tetrahedral molecular structure, resulting in a trigonal pyramidal molecule. The dipole moment of P-Cl bonds does not cancel out as it does in asymmetric PCl3 molecules.
Is pcl3 polar
Phosphorus compounds are very different and inflammable in nature. Phosphorus trichloride has the chemical formula PCl3. All atoms belong to the non-metal family group in the periodic table and possess high electronegativity values. In this blog post, we are going to discuss the polarity of PCl3 in a detailed manner. PCl3 is commonly appearing at ordinary temperatures and pressures, it exists as a liquid with a yellowish texture. PCl3 contains one phosphorus atom and three chlorine atoms. Phosphorus trichloride PCl3 is corrosive to biological tissue and metals, and it can also cause fires when it comes into contact with wood, cotton, and other materials. The phosphorus atom stays the center of the molecule and the remaining three chlorine atoms. Because of the tetrahedral structural form of phosphorus trichloride PCl3. Chlorine has atomic number 17 in the modern periodic table and seven outermost valence shell electrons. It comes under the halogen family group in the periodic table. Similarly, phosphorus has atomic numbers 15 and five outermost valence shell electrons. Total electron counting, only two electron excess in chlorine as compared with phosphorus.
You might also learn from: Is N2 Polar?
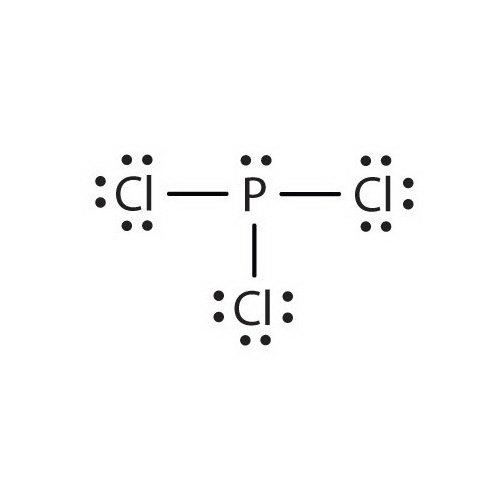
And how can you say that PCl3 is a polar molecule? Note: If you want to know the steps of drawing the PCl3 lewis dot structure, then visit this article: PCl3 lewis structure , Or you can also watch this short 2 minute video. And we also have to check the molecular geometry of PCl3. You can see the electronegativity values of Phosphorus P and Chlorine Cl atoms from the periodic table given below. Hence, the P-Cl bond is a polar covalent bond. But wait, we also have to look at the molecular geometry of PCl3 to know whether it has a symmetric shape or not. Have a look at this 3D structure of PCl3.
And how can you say that PCl3 is a polar molecule? Note: If you want to know the steps of drawing the PCl3 lewis dot structure, then visit this article: PCl3 lewis structure , Or you can also watch this short 2 minute video. And we also have to check the molecular geometry of PCl3. You can see the electronegativity values of Phosphorus P and Chlorine Cl atoms from the periodic table given below. Hence, the P-Cl bond is a polar covalent bond. But wait, we also have to look at the molecular geometry of PCl3 to know whether it has a symmetric shape or not. Have a look at this 3D structure of PCl3. The Phosphorus atom P is at the center and it is surrounded by 3 Chlorine atoms Cl.
Is pcl3 polar
Phosphorus trichloride is a chemical substance known by its chemical formula PCl3. It exists as a colorless to yellow fuming liquid and considered to be toxic in nature. Many of you may have a question regarding whether PCl3 is a polar substance or not. In this article, I will answer this and will cover its properties and applications. PCl3 is a polar molecule because of its tetrahedral geometrical shape having a lone pair on Phosphorus atom and the difference between the electronegativity of Chlorine 3. At room temperature, it exists in the liquid state and is colorless to yellowish in appearance. It is also considered a toxic substance for living beings. It smells unpleasant similar to HCL. It is volatile in nature and reacts vigorously with water to produce HCl gas.
55 gal fish tank
The formation of a polar molecule is caused by the geometrical structure and the difference in electronegativity value of atoms in the PCl3 molecule. Is Benzoic Acid Polar? It is the heterogeneous reaction, phosphorus in the soft solid phase and chlorine in the gas phase. This is because there are more interactions between the molecules in a polar substance, which require more energy to break when the substance melts or boils. The PCl3 molecule has one central phosphorus atom and three chlorine atoms. The dipole moment of entire PCl3 molecule is 0. Because phosphorus and chlorine belong to the nitrogen and halogen family group in the periodic table, their valence electrons are five and seven respectively. There is greater electron density around one side of the phosphorus atom due to an increase in electronegativity on that side of the molecule. Connect all outside atoms Chlorine to the core central atom phosphorus with three single bonds in this stage. To complete its octet , the chlorine atom requires 8 electrons in its outermost valence shell. As a result of this, the dipole moment of the P-Cl bond is non zero, and the dipoles of both P-Cl bonds are not negated due to the tetrahedral structure. Because they generate electrical repulsion among the PCl3 molecule, lone pairs have deformed tetrahedral shapes of the PCl3 molecule. We will response as soon as possible.
Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries.
Polarity of Acetaminophen. Chlorine and phosphorus have seven and five valence electrons in their outermost shell respectively. The formal charge on phosphorus of PCl3 molecule is zero. Your email address will not be published. Learn how your comment data is processed. Mar 6, Is silicon dioxide in supplements and food safe? This results in a bent structure that thereby unequally distributes charge throughout the molecule inducing a permanent dipole. PCl 3 is a polar molecule because the P-Cl bond is polar, and the three bonds are not equivalent to the lone pair which causes an asymmetrical distribution of bonding electrons in the molecule. The dipole moment of PCl3 molecule is 0. We also have two valence electrons to spare in the PCl3 molecule. If swallowed, immediately get medical attention as soon as possible.

0 thoughts on “Is pcl3 polar”